- Vaccination in adult patients with chronic lung diseases
In Switzerland, additional vaccinations against influenza, COVID-19, Streptococcus pneumoniae and varicella zoster virus (VZV), are recommended for patients with chronic lung diseases such as COPD, asthma or interstitial lung disease, since infectious diseases often lead to exacerbation of lung diseases resulting in increased disease burden and mortality. In this review we give an overview on recommended vaccinations for patients with chronic lung diseases, also including vaccinations against pertussis and RSV, which are recommended in international guidelines. While continuous development of vaccines against S. pneumoniae has given rise to high-valency vaccines covering up to 68% of S. pneumoniae variants in individuals aged ≥65 years, vaccination rates in this age group remain low in Switzerland (<10% in 2020). Vaccination rates are higher for influenza, and particularly high-dose vaccines account for high vaccination efficacy in years of low strain matching in individuals at risk. Although mortality of COVID-19 decreased since the emergence of the first SARS-CoV-2 variant, patients with chronic lung disease are still at increased risk for exacerbation, unless vaccinated with variant-adjusted vaccines. VZV and Bordetella pertussis vaccination has also significantly countered reactivation and infection rates, respectively, and subunit vaccines against VZV show long duration. However, pertussis vaccination is still limited by its fast waning. A glimpse into the future presumes the introduction of new higher-valence vaccinations against S. pneumoniae, and several types of RSV vaccines are expected to enter the Swiss market soon.
Key words: Vaccine efficacy, chronic lung diseases, viral infections, exacerbation prevention
Introduction
Chronic lung diseases present a significant health and economic burden worldwide (1). Exacerbations of chronic lung diseases particularly pose a risk for patients with pulmonary disorders, as they can lead to functional impairment, severe pneumonia, hospitalization, and death (2). For chronic obstructive pulmonary disease (COPD), repeated acute exacerbations may lead to an accelerated decline in lung function, impaired quality of life, disease progression, and higher mortality. Thus, COPD exacerbations account for 50% to 75% of COPD-associated health care costs (3). Patients with immunosuppression and/or lung transplantation, asthma, COPD or interstitial lung disease (ILD) are at increased risk of complicated infections (2, 3). Indeed, a majority of acute exacerbations of chronic lung diseases are caused by infections, particularly viral ones (4, 5). Viral detection rates range from 22-64%, and rhinoviruses are reported to be the most commonly detected viral triggers (up to 60%), followed by influenza (up to 36%) and respiratory syncytial virus (RSV; up to 28%) with other viral infections reported less frequently (parainfluenza, human metapneumovirus [HMPV], coronaviruses, adenoviruses) (3). Bacterial co-infections, which occur in about 6-27% of cases, may prolong hospitalization even further, and lead to more severe impairments of lung function (3, 6). Thus, prevention of infections through vaccination is a key management concept to reduce acute infection-driven exacerbations and associated worsening of chronic lung disease (7). Since influenza and Streptococcus pneumoniae lead to increased hospitalization rates and mortality in patients with chronic lung diseases, current international guidelines, as well as the Swiss vaccination plan, recommend influenza and pneumococcus vaccinations in this patient group (7, 8, 9). Similarly, a vaccination against the Severe Acute Respiratory Syndrome coronavirus 2 (SARS-CoV-2) is recommended for individuals at risk in Switzerland (8, 9). Reflecting the fact that other viral infections such as RSV may also contribute to exacerbations, and show similar or even high-er mortality rates than influenza, the Global Initiative for Chronic Obstructive Lung Disease (GOLD) recommends a vaccination with existing RSV vaccines in all patients with COPD (9, 10, 11). Correspondingly, the Federal Office of Public Health (FOPH) has most recently released a recommendation for the RSV vaccination in individuals ≥ 75 years of age and for individuals at high risk of complications, including those with chronic lung disease, above the age of 60 years (12).
This article aims to summarize data on vaccine efficacy (VE) for populations with chronic lung diseases and provides a glimpse into future vaccination options for individuals at risk.
Chronic pulmonary diseases
Chronic obstructive pulmonary disease (COPD)
COPD is the third leading cause of death worldwide, accounting for 3.23 million deaths in 2019 (7, 13). In Switzerland, around 400 000 individuals are affected by COPD (14). It is the 4th most prevalent cause of death in Switzerland, and its incidence is rising (15, 16). COPD affects smokers in particular. However, it can also develop in non-smokers and is characterized by chronic inflammation of lung tissue and loss of lung function, often caused by exacerbations (2). COPD puts a high burden on patients and healthcare systems, with exacerbations causing a decreased quality of life and a negative impact on survival prognosis. In the context of the high burden of COPD, prevention of exacerbation frequently triggered by viral infections is paramount to improve the prognosis of the disease (3).
Asthma
Asthma is a heterogeneous pulmonary inflammation characterized by fluctuating bronchial hyper-responsiveness and variable airflow obstruction. Globally, about 300 million individuals of all ages are affected. This amounts to every 14th person in Switzerland, and incidences are increasing (2, 17). According to the hygiene hypothesis, the increasing prevalence of asthma may be due to a lower incidence of infections and thus less challenges for the immune system (18). However, other theories claim that RSV or rhinovirus infection early in life may also be associated with a higher risk of developing asthma later in life (19, 20).
Interstitial lung disease (ILD)
The term ILD encompasses a wide range of fibrotic and inflammatory lung diseases, the prevalence of which is difficult to estimate due to the lack of standardized definitions (21). Thus, less evidence exists regarding the role of viral infections in the acute exacerbation of fibrotic lung disease (2). However, one report recorded a 30-day inpatient mortality of 20.6% after a viral infection in selected patients with ILD emphasizing the importance of early infection prevention also in this patient population (22). Mortality rates may however vary between different forms of ILD: for example, acute exacerbation of idiopathic pulmonary disease is associated with very high in-hospital mortality (>50%), as opposed to desquamative interstitial pneumonia, which generally comes with a better prognosis (23, 24).
Swiss Vaccination plan for the general population and specific groups
In Switzerland, there are currently 11 recommended basic vaccinations starting in childhood: tetanus, diphtheria, pertussis, poliomyelitis, Haemophilus influenzae type B, hepatitis B, S. pneumoniae, varicella zoster virus (VZV) and the trivalent vaccine against measles, mumps, and rubella. Vaccination against human papilloma virus follows in adolescence. Additional vaccinations in adulthood are recommended against herpes zoster, influenza, and pneumococcus and it is highly encouraged to stay up to date with all vaccinations according to the Swiss vaccination schedule – see link at the end of the article (8).
For at risk groups (incl. elderly and patients with cardiovascular, respiratory or other comorbidities), the Swiss vaccination plan recommends a yearly vaccination against influenza and coronavirus disease 19 (COVID-19) as well as a single dose vaccination against S. pneumoniae and a two-dose vaccination against herpes zoster for individuals aged ≥50 years depending on the classification of the lung disease (8). Indeed, COPD and asthma may be independent risk factors for herpes zoster. Since November 2024, the RSV vaccination is explicitly recommended for individuals aged ≥75 and individuals at high risk aged ≥60, in particular those with chronic lung disease (12).
International guidelines on vaccinations in COPD
The GOLD guidelines, which focus on individuals with COPD, have similar recommendations. However, they do not only emphasize the need for vaccination against influenza, COVID-19, S. pneumoniae, and herpes zoster (in individuals aged ≥50 years), but also RSV vaccination for individuals aged ≥60 years and pertussis vaccination in individuals with chronic lung diseases (9).
In the following chapter, incidences of several important infections in patients with chronic lung diseases as well as evidence for vaccination recommendations in this population will be summarized. Our review is limited to adults.
Pneumococcus vaccination
Streptococcus pneumoniae can cause parenchymal infections in many organs, particularly in the lower respiratory tract, and is a major cause of community-acquired pneumonia (CAP) (25). Exacerbations of COPD in combination with CAP are associated with reduced survival rates and an increased likelihood of subsequent exacerbations (26). Particularly, adults >65 years are affected by invasive pneumococcal disease (IPD) with highly increased incidence in those adults with at risk conditions such as asthma, COPD, and cardiovascular comorbidities. Indeed, two-thirds of pneumococcal disease cases are found in 25% of the population with high-risk and at-risk conditions (25, 27). The reported odds ratios from different cohorts to develop IPD in patients with chronic respiratory diseases range between 1.3 and 4.7 compared to healthy individuals (28). These numbers are concerning, since exacerbations of chronic lung disease with concomitant pneumonia are associated with worse outcomes compared to exacerbations without pneumonia. The length of hospital stay is prolonged (7 vs. 4 days), admission rates to intensive care unit (ICU) are higher (12.5% vs. 7.7%; hazard ratio [HR]: 1.63), there is a higher need for mechanical/non-invasive ventilation (6.9%/9.7% vs. 3.3%/6.7%; HR: 2.10/1.45), and both 30-day mortality in first-time cases (12.1% vs. 8.3%; HR: 1.20) as well as mortality at second exacerbation (15% vs. 10.2.0%; HR: 1.14) are increased (29).
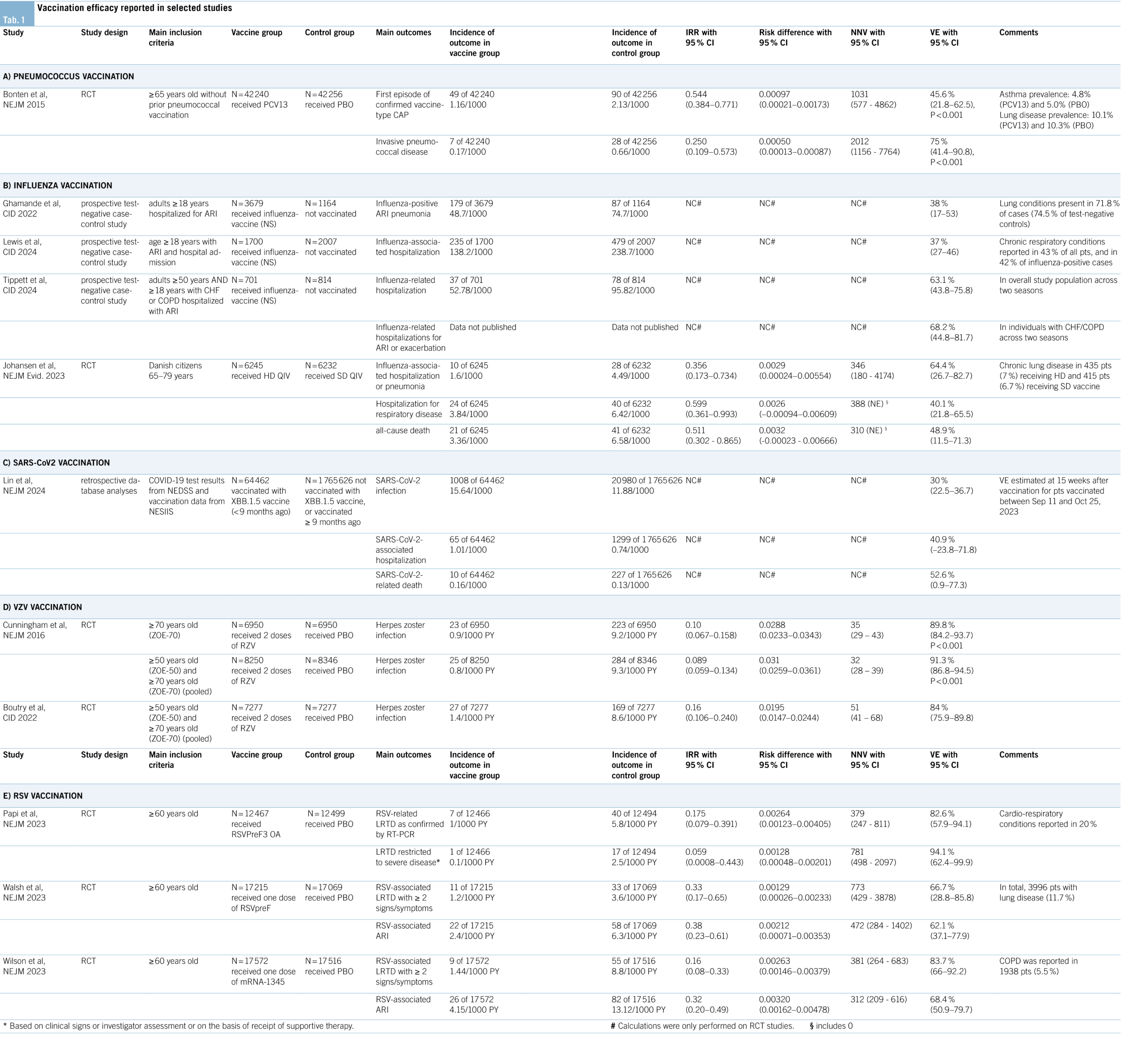
A study from 2005 estimated the incidences for pneumococcal infection at 8.8/100,000 in healthy adults and 62.9/100,000 in adults with chronic lung disease (30). How-ever, more recent data from England reported declining incidences probably due to the introduction of new vaccines in the previous decade (25). In Switzerland, pneumococcal conjugate vaccines (PCVs) are the only pneumococcal vaccines recommended since 2014. They are more effective than the previously used 23-valent pneumococcal polysaccharide vaccine (since 2014, PPV23 [Pneumovax®-23] is not recommended in Switzerland) due to many advantages (31, 32). PCVs elicit a T-cell dependent immune response, are effective also in children under the age of 2 years, generate immune memory, a booster effect, and herd immunity. In addition, they have a higher efficacy in risk-groups compared to the former PPV23 (32, 33). The efficacy of PCV13 vaccination (Prevenar 13®) was tested in the CAPiTA study (details see Table 1A) and suggests a number needed to vaccinate (NNV) of 1031 to prevent one case of CAP and a NNV of 2041 to prevent one case of IPD (33, 34). Since 2023, pneumococcal vaccination with a PCV is recommended for all individuals ≥ 65 years (in addition to children and adults with risk factors) by the FOPH, the Federal commission for Vaccinations, and also by international guidelines (8, 9, 35). Beyond PCV13 (33), the newer higher valency vaccines PCV15 (Vaxneuvance®) (36) and PCV20 (Prevenar 20®) (37) have been recently introduced in Switzerland and approved for use in individuals ≥65 years (35). High valency PCVs are important due to serotype replacement in recent years towards non-vaccine serotypes causing an increasing proportion and incidence of pneumococcal disease (38). In Switzerland, there is a considerable difference in the percentage of covered serotypes between the available vaccines in 2023 – 31% (PCV13), 40% (PCV15), and 68% (PCV20) respectively (39). Even though risk for pneumonia, exacerbation, and death are highly elevated in individuals with chronic lung diseases, the awareness for pneumococcal infections and the requirement for PCV vaccination is still rather low in Switzerland. The vaccination rate among individuals with asthma or chronic pulmonary disease was estimated at about 14.8% in 2020, and even lower at 9.6% in those aged 65 to 85 years, leaving many patients at risk unprotected (40).
Influenza vaccination
The influenza virus infects about 10-20% of the global population each year, causing 3-5 million hospitalizations annually. It is a leading cause for mortality, particularly in individuals at risk (41). Influenza also causes a high economical and individual burden in Switzerland, leading to a seasonal average of 4944 (standard error: ± 785) influenza-caused hospitalizations and direct medical costs of up to 77.3 million euros per season (42). A less appreciated danger of the virus lies in complications affecting the cardiovascular system (myocarditis, heart failure), the central nervous system (stroke, encephalitis) or other organs such as the kidneys and liver (acute kidney/liver injury). For example, it is estimated that up to 13% of hospitalized adult patients with influenza develop myocarditis (41). Another study reported acute cardiac injury within the first 3 days of influenza infection in 24% of high-risk patients (43, 44). Several meta-analyses and reviews have summarized evidence that a vaccination against influenza attenuates the severity of an influenza infection in individuals at risk and can prevent such complications. Evidence supporting flu vaccination is mostly based on case-control studies and cohort studies. Indeed, a meta-analysis identified only four randomized controlled trials (RCTs) including patients with COPD. Vaccination against influenza showed long-term benefits for patients with COPD regarding influenza-related respiratory infections, number of exacerbations, hospitalization rates, all-cause mortality, and respiratory mortality (45). Further, it was shown in a meta-analysis of RCTs (vaccination versus placebo) that influenza vaccination was able to decrease the rate of severe adverse cardiovascular events (RR: 0.64; 95% CI: 0.48-0.86) particularly in high-risk patients, giving a favorable NNV of 58 (46). Moreover, vaccination leads to a decrease in stroke occurrence (odds ratio [OR]: 0.81; 95% CI: 0.77-0.86), a reduction of mortality among stroke patients (OR: 0.50; 95% CI: 0.37-0.68) and a decrease of stroke occurrence in COPD (OR: 0.70; 95% CI: 0.61-0.81) (47). Additionally, all-cause mortality was reduced by 4.6% and hospitalization rates for pneumonia and influenza were reduced by 8.5% in individuals ≥65 years, and by 12.4% in individuals aged 50-64 years
(48, 49). Besides reducing direct health risks in a vulnerable population, influenza vaccination may also decrease antibiotic use due to prevention of CAP: Seasonal influenza vaccination averts about 1014.7 (95% CI: 803.3-1219.7) million defined daily doses of antibiotics (50).
Recent studies on the efficacy of different available influenza vaccines are summarized in table 1B. Notably, the US HAIVEN study reported an adjusted VE of 38% (95% CI: 17-53) against hospitalization for influenza-associated pneumonia, varying between strains (51). A multicenter study from the US showed a similar VE of 37% (95% CI: 27–46) against hospitalization, also reporting variations depending on strain and age (52). Different results were reported in another prospective study from the US, which found that the adjusted VE against influenza-related hospitalizations was 63.1% (95% CI: 43.8-75.8) in the pooled study population, and 68.2% (95% CI: 44.8-81.7) against hospitalization for exacerbation or acute respiratory infection (ARI) among those with congestive heart failure (CHF) or COPD (53). However, VE is estimated to have varied annually from 19% – 60% in the last 15 years, mainly due to differences in matching of vaccine strains with circulating strains (54). High-dose (HD) vaccines improve VE, particularly in seasons of low matching between the vaccine and the circulating virus strains and in older individuals or those at risk (55). Current Swiss recommendations emphasize the importance of influenza vaccination in individuals at risk, including those with chronic lung diseases (56). In Switzerland, the quadrivalent inactivated vaccines Fluarix Tetra® (57) and Vaxigrip Tetra® (approved from the age of 6 months) (58) are available and reimbursed for individuals ≥65 years and for all individuals with at least one risk factor (56). Recently, the HD quadrivalent influenza vaccine showed a relative VE of 64.4% regarding the prevention of hospitalizations due to influenza or pneumonia and of 48.9% regarding all-cause death, corresponding to a NNV of 346 and 311 respectively (see Table 1B for details) (59). Accordingly, the HD Efluelda® vaccine (60) is available in Switzerland for individuals ≥65 years and reimbursed in all individuals ≥75 years or ≥65 with at least one risk factor (56). Compared to the aforementioned pneumococcal vaccination, the rate of influenza vaccination among people with chronic lung diseases is higher in Switzerland, reaching 21.6% (95% CI: 18.68-24.85) in all age groups and 49.85% (95% CI: 41.61-58.09) in those aged ≥65 (61). For the coming influenza season 2024/2025, the US has decided on a transition back to the trivalent vaccine, as the B/Yamagata strain (included in the quadrivalent vaccines) is no longer circulating (62).
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccination
Despite the decreasing media interest in SARS-CoV-2, the rates of the circulating virus are still high in 2024 (63). With the emergence of new variants, the mortality of a SARS-CoV-2 infection decreased, but is still 35% higher compared to an influenza infection (HR: 1.35; 95% CI: 1.10-1.66) (64). During earlier waves, the rate of severe COVID-19 was significantly higher in individuals who had a lung transplantation (OR: 4.62; 95% CI: 2.71-7.89) or chronic lung disease (OR: 2.11; 95% CI: 1.36-3.30) compared to the general population (65). Although severe COVID-19 cases have decreased in the Omicron era, individuals with asthma and COPD still had a significantly higher risk for developing severe COVID-19 (HR: 1.31; 95% CI: 1.10-1.55 and HR: 1.36; 95% CI: 1.12-1.66, respectively), but booster vaccinations reduced this risk (66). Administration of ≥3 doses of vaccination conferred a significantly reduced risk for a severe infection (OR: 0.35; 95% CI: 0.21-0.60) (65).
A study on the VE of SARS-CoV-2 vaccines is summarized in table 1C. In general, XBB.1.5 vaccines against omicron subvariants showed limited duration against preventing infection, with a VE of 52.2% (95% CI: 44.6-58.7) after 4 weeks, and 32.6% (95% CI: 28.1-36.8) after 10 weeks. However, VE against hospitalization and death was sustained over a longer period (67). Thus, protection against SARS-CoV-2 infection itself is only modest, but protection against severe COVID-19 leading to hospitalization remains high after vaccination. Accordingly, the FOPH currently recommends a SARS-CoV-2 vaccination for all individuals at risk, ideally with variant-adjusted vaccines (8). Similarly, also the GOLD-guidelines recommend a yearly COVID-19 vaccination for patients with COPD (9). Currently, the mRNA vaccines Comirnaty® (68) and Spikevax® (69) are available and approved for use in Switzerland. In general, the FOPH recommends the use of an mRNA vaccine targeting the current SARS-CoV-2 variants, regardless of previously administered vaccinations (70).
Varicella-zoster virus (VZV) vaccination
VZV causes varicella (chickenpox) and herpes zoster (shingles). Varicella typically manifests in childhood, characterized by a highly contagious vesicular rash and mild fever. Herpes zoster occurs due to reactivation of latent VZV, usually in older adults or immunocompromised individuals causing a painful, localized rash and severe nerve pain (postherpetic neuralgia) (71, 72). Worldwide, almost 84 million people are affected annually, leading to around 14,500 deaths per year. Although death rates appear to be rather low, the disease burden in affected people is high (72). The risk for developing herpes zoster is 24% higher in patients with asthma, and 41% higher in patients with COPD. This risk is further increased by the use of corticosteroid medication. Moreover, an exacerbation of COPD seems to be coupled with herpes zoster appearance and there is a higher risk for herpes zoster complications (postherpetic neuralgia, zoster ophthalmicus) in patients with asthma and COPD (73). Even though incidences are rising due to increased aging of the population, disease burden and death rates have decreased during the last decades. This is mostly attributable to vaccination, in particular with subunit vaccines (Shingrix®) (72, 74). Table 1D provides a summary on the VE of this recombinant VZV vaccination from two randomized controlled trials (RCTs). One study suggests a NNV of 35 (95% CI: 29-43) to prevent herpes zoster infection in ≥70-year-old patients, and a NNV of 32 (95% CI: 28-39) in ≥50-year-old patients (75). A 7-year follow-up on the cohort of patients ≥50 years reported a NNV of 51 (95% CI: 41-68) (76). In contrast, the protection of the live, attenuated vaccine waned over this time period (77). In Switzerland, vaccination with the recombinant subunit vaccine Shingrix® (78) is recommended for individuals aged ≥ 65 years, for individuals aged ≥ 50 years with severe asthma, COPD or immunodeficiency, and for individuals aged ≥ 18 years with severe immunosuppression (8).
Pertussis vaccination
Pertussis, or whooping cough, is a highly contagious disease characterized by severe coughing. It is caused by the bacterium Bordetella pertussis and affects about 50 million people worldwide every year, causing 300,000 deaths annually (79). Incidence in healthy people amounts to 0.5 per 100,000 and is significantly increased among patients with COPD (2.47 per 100,000; incidence rate ratio [IRR]: 4.94; 95% CI: 4.0-6.1;). Pertussis incidence in asthma patients is even higher with 3.35 per 100,000 (IRR: 6.70; 95% CI: 5.7-7.9) (79). Thus, specifically in patients with chronic lung disease, pertussis events cause a significant increase in health care resource utilization and direct medical costs (80). With the introduction of vaccines, mortality was significantly reduced, but there are still surges of the disease, which may be countered with up-to-date booster vaccinations (81). The surges may be attributable to the rather fast waning of protection from acellular vaccine as reported by a study on the Tdap-vaccines (tetanus, diphtheria, acellular pertussis) Boostrix® (82) and Adacel® (83). While VE was 75.3% (95% CI: 55.2-86.5) within 1 year, it decreased to 11.9% (95% CI: -11.1-30.1) within 4-5 years (84).
The FOPH currently recommends a basic pertussis vaccination in infants and boosters in childhood and adolescence. Moreover, a booster vaccination with either Boostrix® (82) or Adacel® (83) is recommended in adults who are in contact with infants and pregnant women. However, patients with COPD and asthma are not particularly mentioned (8). Also GOLD recommends a pertussis booster only in COPD patients who were not vaccinated in adolescence (9).
Future perspectives in Switzerland
In this section, we aim to provide a view of the future vaccination landscape in Switzerland, with a focus on new pneumococcus vaccines with higher serotype coverage and the first RSV vaccines already and soon to be available in Switzerland.
New pneumococcus vaccines
Currently, PCV15 and PCV20 are recommended for use in Switzerland (35). The US Federal Drug Administration (FDA) recently approved PCV21 for people aged ≥65 years, which represents a new concept of PCVs as it targets the majority of serotypes which currently affect adults. PCV21 does not include all pediatric serotypes, but it currently covers up to 85% of serotypes in those aged ≥65 years (85, 86). The U.S. Advisory Committee on Immunization Practices has already recommended this PCV21 as an option for adults. This recommendation also applies to individuals who had already received PCV13 in the past (86). Due to the coverage of 11 different serotypes, it is expected to bring benefits in terms of quality of life for the older population and a potential benefit for patients at risk for IPD (87). This approval is based on a recent study on the PCV21 vaccine in adults, which had proven a good tolerability and safety profile, while showing a non-inferior response to all included serotypes in comparison to previous PCV vaccinations covering fewer serotypes (88). Additionally, there are ongoing studies on a novel 24-valent PCV, which aims to increase even further serotype coverage (89).
Respiratory syncytial virus (RSV) vaccines
RSV is an RNA-virus, with its infections peaking in the winter months, causing a range of respiratory tract symptoms, and sometimes even pneumonia. Severe cases affect mostly infants, young children, and the elderly, leading to higher mortality in those age groups (90, 91). There are already two approved protein-based RSV vaccines available in Switzerland (92), and a third mRNA-based vaccine is currently in the approval process. Vaccination recommendations for older individuals and patients at high risk for complications, including those with chronic lung disease, have recently been published by the FOPH (93). Similarly, the GOLD guidelines recommend RSV vaccination for patients with COPD (9). This recommendation is based on the fact, that RSV is associated with more severe disease outcomes in comparison to influenza or SARS-CoV-2, even though less people are hospitalized with RSV (94). However, chronic lung diseases are among the major predictors for hospitalization of patients, infected with RSV (95). A recent modeling study reported a 2- to 4-fold increased risk of hospitalization for adults with COPD and RSV infection and a 1.5- to 3-fold increased risk for adults with asthma and RSV infection (96). Moreover, hospitalization due to RSV infection is associated with acute cardiac events, particularly in patients at risk (97).
In recent years, many RSV-vaccinations were studied in clinical trials, aiming to elicit an immune response against the RSV fusion protein F in its prefusion conformation (preF) (98). Besides the adjuvant RSVPreF3-antigen vaccination mentioned above (99), there is also a bivalent vaccine including the preF from both RSV A and B (100) as well as an mRNA vaccine (101) already available in some markets, albeit not approved in Switzerland yet. Several RCTs testing those different vaccines are summarized in table 1E. For the AS01E-adjuvanted RSV preF based candidate vaccine (RSVPreF3 OA) a NNV of 379 (95% CI: 247-811) was reported regarding the prevention of RSV-related lower respiratory tract disease (LRTD) (99). For the bivalent RSV preF based vaccine (bivalent RSVpreF) a NNV of 773 (95% CI: 429-3878) for RSV-associated LRTD with ≥2 signs/symptoms, and a NNV of 472 (95% CI: 284-1402) for RSV-associated ARI was reported (100). Lastly, the mRNA vaccine mRNA-1345 show-ed a NNV of 381 (95% CI: 264-683) against RSV-associated LRTD with ≥2 signs/symptoms and a NNV of 312 (95% CI: 209-616) for RSV-associated ARI (101). The immunization against preF was able to maintain a high VE against LRTD over a period of at least two to three seasons (102, 103). While the VE regarding severe RSV and LRTD is well studied in current RSV vaccines, data on VE regarding the prevention of hospitalizations is still limited. In the US, three RSV vaccines (one adjuvant RSVPreF3, one bivalent RSVpreF, and the mRNA vaccine) are already approved for adults ≥60 years, and the first safety data were recently presented (104). So far, injection site and systemic reactions were more frequently reported among patients receiving the adjuvant RSVPreF3 vaccine compared to the available bivalent RSVpreF. How-ever, the estimated rates for Guillain-Barré syndrome (GBS), which had been raised as a safety concern, were higher in people vaccinated with the bivalent vaccine (4.4 per 1 million administered doses) compared to the adjuvant one (1.8 per 1 million doses administered) (104). Even though GBS rates were more commonly reported than initially expected, the high efficacy in reducing severe RSV cases and exacerbations of chronic lung disease still suggests the importance of those RSV vaccines in at-risk patients.
Discussion
In summary, the Swiss vaccination plan provides specific recommendations for the vaccination of individuals at risk, including patients with chronic lung diseases (8). Still, the vaccination rate among patients with chronic lung diseases, particular for S. pneumoniae, remains low in Switzerland, highlighting the need for more awareness in clinics and among general practitioners (40). Moreover, due to serotype replacement in pneumococcal disease observed in recent years, recommendation for newer PCVs covering more serotypes, especially for individuals at risk, should be made (38). This may include the PCV21 in the near future, as it has recently been approved by the FDA (86). While the GOLD guidelines have already recommended RSV and pertussis vaccinations for patients with COPD, the recommendations regarding RSV were only recently anchored in the Swiss recommendations (8, 9, 12). This recent update reflects the potential severity of an RSV infection for patients with chronic lung diseases (94). Indeed, the FDA has already approved three RSV vaccines in the last years (104). Also, Switzerland has recently introduced two vaccines on the market for adults aged ≥60 years (93). Several impor-tant questions remain regarding RSV vaccination, such as the efficacy of repeated vaccination, duration of response and most importantly the effectiveness in populations with comorbidities (105). Moreover, safety and immunogenicity of coadministration with for example influenza vaccines are still under investigation. Current studies suggest that coadministration is probably acceptable, even though a slight reduction in RSV antibody responses was observed (106). In general, even though RSV vaccinations are associated with certain risks such as GBS, those are outweighed by the successful prevention of exacerbations and cardiovascular events in individuals at risk (107). This goes in line with the protective properties of for example influenza and S. pneumoniae vaccines, which have shown to reduce the risk for myocardial infarction and cardiovascular additionally to pulmonary exacerbations (107).
Our review has several limitations. It is not a systematic review and meta-analysis, as we incorporated only selected studies evaluating the NNV and VE of various vaccin-es recommended for patients with chronic lung diseases. Furthermore, most of those vaccination studies were not explicitly performed in patients with chronic lung diseases. While some of the mentioned studies assessed the percentage of patients with chronic lung diseases, COPD and asthma in particular, there was almost no record of ILD. In general, a lot of the recommendations for vaccination in patients with chronic lung diseases focus on COPD (9). Although there are some studies on the role of infection-driven exacerbations in asthma, data on the impact of res-piratory infections on the exacerbation of ILD are scarce (21).
In conclusion, aiming for a higher vaccination rate among individuals with chronic pulmonary diseases is crucial in preventing exacerbations and thus morbidity and mortality in this vulnerable population. The introduction of new and more effective vaccines, such as updated PCVs and RSV vaccines in Switzerland as well as the constant variant-adjustment of influenza and SARS-CoV2 vaccines will be pivotal in ensuring protection in comorbid patients in the future. Strategies to improve vaccination rates may include the identification of drivers of and barriers to vaccinations to make informed decisions, as well as patient education and training for healthcare providers and national authorities (108).
Abbreviations
ARI acute respiratory illness
CAP community acquired pneumonia
CI confidence interval
CHF congestive heart failure
COPD chronic obstructive pulmonary disease
COVID-19 coronavirus disease 19
FEV1 Forced Expiratory Volume in one second
FOPH Swiss federal office of public health
GOLD Global initiative for chronic obstructive lung disease
HD high dose
HMPV human metapneumovirus
HR hazard ratio
ILD interstitial lung disease
IPD invasive pneumococcal disease
IRR incidence rate ratio
LRTD lower respiratory tract disease
mRNA-1345 mRNA-based RSV vaccine encoding the stabilized RSV prefusion F glycoprotein
NC not calculable
NE not estimable
NEDSS Nebraska Electronic Disease Surveillance System
NESIIS Nebraska State Immunization Information System
NS not specified
NNV number needed to vaccinate (rounded to unit)
OR odds ratio
PBO placebo»
PCV pneumococcal conjugate vaccine
PPV pneumococcal polysaccharide vaccine
PY person years
QIV quadrivalent influenza vaccine
RCT randomized controlled trial
RR risk ratio
RSV respiratory syncytial virus
RSVpreF bivalent RSV prefusion F protein-based vaccine
RSVPreF3 OA AS01E-adjuvanted RSV prefusion F protein–based vaccine
RZV glycoprotein E (gE)-based adjuvanted recombinant zoster vaccine
SARS-CoV-2 severe acute respiratory syndrome coronavirus 2
SD standard dose
VE vaccine efficacy
VZV varicella zoster virus
Medicine & University Affairs
Cantonal Hospital Baselland
Medical Faculty
University of Basel, Basel, Switzerland
Klinik für Infektiologie/Spitalhygiene
KSSG und Ostschweizer Kinderspital
St. Gallen
Service de pneumologie
Centre Hospitalier du Valais Romand
Hôpital du Valais
Sion
Faculté de médecine, Université de Genève
Service de pneumologie
Hôpitaux universitaires de Genève
Switzerland
Department of Pneumology
University Hospital Zurich
Réseau hospitalier neuchâtelois
Neuchâtel
Switzerland
Ente Ospedaliero Cantonale
Lugano
Switzerland
Department of Pneumology
Cantonal Hospital St. Gallen
Chefärztin Pneumologie
Hochgebirgsklinik Davos
Herman-Burchard-Strasse 1
7265 Davos Wolfgang
tsogyal.latshang@hgk.ch
Department of Pneumology
University Hospital Bern
Bern
Department of Internal Medicine
Cantonal Hospital Winterthur
Winterthur
Switzerland
University of Zurich
Zurich
Switzerland
Lausanne University Hospital
(CHUV) and University of Lausanne
Lausanne
Switzerland
Facharzt Allgemeine Innere Medizin und Pneumologie
Klinischer Professor für Innere Medizin Universität Basel
Chief Medial Officer und Leiter Universitäres Instiut Innere Medizin
Kantonsspital Baselland
joerg.leuppi@ksbl.ch
The manuscript was financially supported by GSK AG Switzerland and Moderna AG Switzerland. The sponsors did not have any influence on the content of the scientific review.
Jörg D. Leuppi has received unrestricted grants from AstraZeneca AG Switzerland, GSK AG Switzerland, OM Pharma SA Switzerland, and Sanofi AG Switzerland. Werner Albrich received funding from Swiss National Science Foundation (33IC30_201300), Cantonal Hospital St. Gallen, OM Pharma, FUNGINOS, Gilead, received payment for lectures and presentations by Pfizer, GSK, MSD, Gilead, paid to his institution, received payment for travel to meetings from Pfizer, GSK, Gilead, paid to his institution, and participated in the advisory boards of MSD, Sanofi, Pfizer, GSK, OM Pharma, Moderna, Aurovir Pharma, and Janssen.
1. Iheanacho I, Zhang S, King D, Rizzo M, Ismaila AS. Economic Burden of Chronic Obstructive Pulmonary Disease (COPD): A Systematic Literature Review. Int J Chron Obstruct Pulmon Dis. 2020;15:439-60.
2. Britto CJ, Brady V, Lee S, Dela Cruz CS. Respiratory Viral Infections in Chronic Lung Diseases. Clin Chest Med. 2017;38(1):87-96.
3. Ritchie AI, Wedzicha JA. Definition, Causes, Pathogenesis, and Consequences of Chronic Obstructive Pulmonary Disease Exacerbations. Clin Chest Med. 2020;41(3):421-38.
4. Kherad O, Kaiser L, Bridevaux PO, Sarasin F, Thomas Y, Janssens JP, et al. Upper-respiratory viral infection, biomarkers, and COPD exacerbations. Chest. 2010;138(4):896-904.
5. Bridevaux PO, Aubert JD, Soccal PM, Mazza-Stalder J, Berutto C, Rochat T, et al. Incidence and outcomes of respiratory viral infections in lung transplant recipients: a prospective study. Thorax. 2014;69(1):32-8.
6. Papi A, Bellettato CM, Braccioni F, Romagnoli M, Casolari P, Caramori G, et al. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am J Respir Crit Care Med. 2006;173(10):1114-21.
7. Simon S, Joean O, Welte T, Rademacher J. The role of vaccination in COPD: influenza, SARS-CoV-2, pneumococcus, pertussis, RSV and varicella zoster virus. Eur Respir Rev. 2023;32(169).
8. Bundesamt für Gesundheit (BAG). Impfplan 2024. https://www.bag.admin.ch/bag/de/home/gesund-leben/gesundheitsfoerderung-und-praevention/impfungen-prophylaxe/schweizerischer-impfplan.html; last accessed October 2024.
9. Global Initiative for Chronic Obstructive Lung Disease; GOLD Report 2024. https://goldcopd.org/2024-gold-report/; last accessed July 2024.
10. Hedberg P, Karlsson Valik J, Abdel-Halim L, Alfvén T, Nauclér P. Outcomes of SARS-CoV-2 Omicron Variant Infections Compared With Seasonal Influenza and Respiratory Syncytial Virus Infections in Adults Attending the Emergency Department: A Multicenter Cohort Study. Clin Infect Dis. 2024;78(4):900-7.
11. National Library of Medicine (NIH). https://clinicaltrials.gov/study/NCT01255410; last accessed: September 2024.
12. Bundesamt für Gesundheit (BAG). Impfempfehlungen gegen Erkrankungen mit dem Respiratorischen Synzytial-Virus (RSV). BAG Bulletin, 47/2024.
13. World Health Organisation (WHO). Global Alliance against Chronic Respiratory Diseases (GARD). https://www.who.int/groups/global-alliance-against-chronic-respiratory-diseases-%28gard%29/; last accessed July 2024.
14. Lungenliga. COPD. https://www.lungenliga.ch/krankheiten-therapien/copd; last accessed July 2024.
15. Bundesamt für Gesundheit (BAG). Chronische Atemwegserkrankungen. https://www.bag.admin.ch/bag/de/home/krankheiten/krankheiten-im-ueberblick/chronische-atemwegserkrankungen.html#-876619191; last accessed October 2024.
16. McLean S, Hoogendoorn M, Hoogenveen RT, Feenstra TL, Wild S, Simpson CR, et al. Projecting the COPD population and costs in England and Scotland: 2011 to 2030. Sci Rep. 2016;6:31893.
17. Lungenliga. Asthma. https://www.lungenliga.ch/krankheiten-therapien/asthma; last accessed July 2024.
18. Strachan DP. Family size, infection and atopy: the first decade of the „hygiene hypothesis“. Thorax. 2000;55 Suppl 1(Suppl 1):S2-10.
19. Sigurs N, Aljassim F, Kjellman B, Robinson PD, Sigurbergsson F, Bjarnason R, et al. Asthma and allergy patterns over 18 years after severe RSV bronchiolitis in the first year of life. Thorax. 2010;65(12):1045-52.
20. Jackson DJ, Gangnon RE, Evans MD, Roberg KA, Anderson EL, Pappas TE, et al. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med. 2008;178(7):667-72.
21. Shah Gupta R, Koteci A, Morgan A, George PM, Quint JK. Incidence and prevalence of interstitial lung diseases worldwide: a systematic literature review. BMJ Open Respir Res. 2023;10(1).
22. Li L, Wang C, Sun L, Zhang X, Yang G. Clinical characteristics and prognostic risk factors of mortality in patients with interstitial lung diseases and viral infection: a retrospective cohort study. J Med Microbiol. 2021;70(11).
23. Kreuter M, Polke M, Walsh SLF, Krisam J, Collard HR, Chaudhuri N, et al. Acute exacerbation of idiopathic pulmonary fibrosis: international survey and call for harmonisation. Eur Respir J. 2020;55(4).
24. Tazelaar HD, Wright JL, Churg A. Desquamative interstitial pneumonia. Histopathology. 2011;58(4):509-16.
25. Parks T, Barrett L, Jones N. Invasive streptococcal disease: a review for clinicians. Br Med Bull. 2015;115(1):77-89.
26. Niu Y, Xing Y, Li J, Shui W, Gu Y, Zhang C, et al. Effect of Community-Acquired Pneumonia on Acute Exacerbation of Chronic Obstructive Pulmonary Disease. Copd. 2021;18(4):417-24.
27. Pelton SI, Shea KM, Weycker D, Farkouh RA, Strutton DR, Edelsberg J. Rethinking risk for pneumococcal disease in adults: the role of risk stacking. Open Forum Infect Dis. 2015;2(1):ofv020.
28. Torres A, Blasi F, Dartois N, Akova M. Which individuals are at increased risk of pneumococcal disease and why? Impact of COPD, asthma, smoking, diabetes, and/or chronic heart disease on community-acquired pneumonia and invasive pneumococcal disease. Thorax. 2015;70(10):984-9.
29. Søgaard M, Madsen M, Løkke A, Hilberg O, Sørensen HT, Thomsen RW. Incidence and outcomes of patients hospitalized with COPD exacerbation with and without pneumonia. Int J Chron Obstruct Pulmon Dis. 2016;11:455-65.
30. Kyaw MH, Rose CE, Jr., Fry AM, Singleton JA, Moore Z, Zell ER, et al. The influence of chronic illnesses on the incidence of invasive pneumococcal disease in adults. J Infect Dis. 2005;192(3):377-86.
31. Current product information of Pneumovax®-23 (PPV23) available at www.swissmedicinfo.ch.
32. Bundesamt für Gesundheit (BAG) und Eidgenössische Kommission für Impffragen (EKIF). Pneumokokkenimpfung: Empfehlungen zur Verhinderung von invasiven Pneumokokken erkrankungen bei Risikogruppen. Stand: Februar 2014.
33. Current product information of Prevenar 13® (PCV13) available at www.swissmedicinfo.ch.
34. Bonten MJ, Huijts SM, Bolkenbaas M, Webber C, Patterson S, Gault S, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med. 2015;372(12):1114-25.
35. Bundesamt für Gesundheit (BAG). Pneumokokken-Impfung neu für alle Personen ab dem Alter von 65 Jahren als ergänzende Impfung empfohlen. BAG Bulletin, 2024.
36. Current product information of Vaxneuvance® (PCV15) available at www.swissmedicinfo.ch.
37. Current product information of Prevenar 20® (PCV20) available at www.swissmedicinfo.ch.
38. Ouldali N, Varon E, Levy C, Angoulvant F, Georges S, Ploy MC, et al. Invasive pneumococcal disease incidence in children and adults in France during the pneumococcal conjugate vaccine era: an interrupted time-series analysis of data from a 17-year national prospective surveillance study. Lancet Infect Dis. 2021;21(1):137-47.
39. University of Bern. Annual Report of the National Center for invasive Pneumococci (NZPn), 2023. Available at: https://www.ifik.unibe.ch/unibe/portal/fak_medizin/ber_vkhum/inst_infekt/content/e39965/e39976/e1098920/e1565106/NZPn_Jahresbericht2023_ger.pdf; last accessed October 2024. .
40. Zens KD, Baroutsou V, Fehr JS, Lang P. Pneumococcal Vaccination Coverage and Uptake Among Adults in Switzerland: A Nationwide Cross-Sectional Study of Vaccination Records. Front Public Health. 2021;9:759602.
41. Sellers SA, Hagan RS, Hayden FG, Fischer WA, 2nd. The hidden burden of influenza: A review of the extra-pulmonary complications of influenza infection. Influenza Other Respir Viruses. 2017;11(5):372-93.
42. Ammann D, Bilger J, Loiacono MM, Oberle SG, Dounas A, Manuel O, et al. Burden of seasonal influenza in the Swiss adult population during the 2016/2017-2018/2019 influenza seasons. Influenza Other Respir Viruses. 2023;17(11):e13218.
43. Kodama M. Influenza myocarditis. Circ J. 2010;74(10):2060-1.
44. Ludwig A, Lucero-Obusan C, Schirmer P, Winston C, Holodniy M. Acute cardiac injury events ≤30 days after laboratory-confirmed influenza virus infection among U.S. veterans, 2010-2012. BMC Cardiovasc Disord. 2015;15:109.
45. Bekkat-Berkani R, Wilkinson T, Buchy P, Dos Santos G, Stefanidis D, Devaster JM, et al. Seasonal influenza vaccination in patients with COPD: a systematic literature review. BMC Pulm Med. 2017;17(1):79.
46. Udell JA, Zawi R, Bhatt DL, Keshtkar-Jahromi M, Gaughran F, Phrommintikul A, et al. Association between influenza vaccination and cardiovascular outcomes in high-risk patients: a meta-analysis. Jama. 2013;310(16):1711-20.
47. Zahhar JA, Salamatullah HK, Almutairi MB, Faidah DE, Afif LM, Banjar TA, et al. Influenza vaccine effect on risk of stroke occurrence: a systematic review and meta-analysis. Front Neurol. 2023;14:1324677.
48. Baxter R, Ray GT, Fireman BH. Effect of influenza vaccination on hospitalizations in persons aged 50 years and older. Vaccine. 2010;28(45):7267-72.
49. Fireman B, Lee J, Lewis N, Bembom O, van der Laan M, Baxter R. Influenza vaccination and mortality: differentiating vaccine effects from bias. Am J Epidemiol. 2009;170(5):650-6.
50. Lewnard JA, Charani E, Gleason A, Hsu LY, Khan WA, Karkey A, et al. Burden of bacterial antimicrobial resistance in low-income and middle-income countries avertible by existing interventions: an evidence review and modelling analysis. Lancet. 2024;403(10442):2439-54.
51. Ghamande S, Shaver C, Murthy K, Raiyani C, White HD, Lat T, et al. Vaccine Effectiveness Against Acute Respiratory Illness Hospitalizations for Influenza-Associated Pneumonia During the 2015-2016 to 2017-2018 Seasons: US Hospitalized Adult Influenza Vaccine Effectiveness Network (HAIVEN). Clin Infect Dis. 2022;74(8):1329-37.
52. Lewis NM, Zhu Y, Peltan ID, Gaglani M, McNeal T, Ghamande S, et al. Vaccine Effectiveness Against Influenza A-Associated Hospitalization, Organ Failure, and Death: United States, 2022-2023. Clin Infect Dis. 2024;78(4):1056-64.
53. Tippett A, Ess G, Hussaini L, Reese O, Salazar L, Kelly M, et al. Influenza Vaccine Effectiveness Pre-pandemic Among Adults Hospitalized With Congestive Heart Failure or Chronic Obstructive Pulmonary Disease and Older Adults. Clin Infect Dis. 2024;78(4):1065-72.
54. Centers for Disease Control and Prevention (CDC). Seasonal Flu Vaccine Effectiveness Studies. https://www.cdc.gov/flu/vaccines-work/effectiveness-studies.htm; last accessed July 2024.
55. Chaves SS, Naeger S, Lounaci K, Zuo Y, Loiacono MM, Pilard Q, et al. High-Dose Influenza Vaccine Is Associated With Reduced Mortality Among Older Adults With Breakthrough Influenza Even When There Is Poor Vaccine-Strain Match. Clin Infect Dis. 2023;77(7):1032-42.
56. Federal Office of Public Health (FOPH). Seasonal flu (influenza). https://www.bag.admin.ch/bag/en/home/krankheiten/krankheiten-im-ueberblick/grippe.html#-2084790127; last accessed October 2024.
57. Current product information of Fluarix Tetra® available at www.swissmedicinfo.ch.
58. Current product information of VaxigripTetra® available at www.swissmedicinfo.ch.
59. Johansen ND, Modin D, Nealon J, Samson S, Salamand C, Loiacono MM, et al. A Pragmatic Randomized Feasibility Trial of Influenza Vaccines. NEJM Evid. 2023;2(2):EVIDoa2200206.
60. Current product information of Efluelda® available at www.swissmedicinfo.ch.
61. Zürcher K, Zwahlen M, Berlin C, Egger M, Fenner L. Losing ground at the wrong time: trends in self-reported influenza vaccination uptake in Switzerland, Swiss Health Survey 2007-2017. BMJ Open. 2021;11(2):e041354.
62. Centers for Disease Control and Prevention (CDC). Influenza (Flu). https://www.cdc.gov/flu/spotlights/2023-2024/trivalent-vaccines-2024-2025.htm; last accessed September 2024.
63. Eawag aquatic research. SARS-CoV-2 in Wastewater. https://sensors-eawag.ch/sars/overview.html; last accessed July 2024.
64. Xie Y, Choi T, Al-Aly Z. Mortality in Patients Hospitalized for COVID-19 vs Influenza in Fall-Winter 2023-2024. Jama. 2024;331(22):1963-5.
65. Solera JT, Árbol BG, Mittal A, Hall V, Marinelli T, Bahinskaya I, et al. Longitudinal outcomes of COVID-19 in solid organ transplant recipients from 2020 to 2023. Am J Transplant. 2024;24(7):1303-16.
66. Wee LE, Tan JYJ, Chiew CJ, Abisheganaden JA, Chotirmall SH, Lye DCB, et al. A Nationwide Cohort Study of Delta and Omicron SARS-CoV-2 Outcomes in Vaccinated Individuals With Chronic Lung Disease. Chest. 2024.
67. Lin DY, Du Y, Xu Y, Paritala S, Donahue M, Maloney P. Durability of XBB.1.5 Vaccines against Omicron Subvariants. N Engl J Med. 2024;390(22):2124-7.
68. Current product information of Comirnaty® available at www.swissmedicinfo.ch.
69. Current product information of Spikevax® available at www.swissmedicinfo.ch.
70. Federal Office of Public Health (FOPH). Information on the COVID-19 vaccination. https://www.bag.admin.ch/bag/en/home/krankheiten/krankheiten-im-ueberblick/coronavirus/covid-19/impfen.html; last accessed October 2024.
71. Pan CX, Lee MS, Nambudiri VE. Global herpes zoster incidence, burden of disease, and vaccine availability: a narrative review. Ther Adv Vaccines Immunother. 2022;10:25151355221084535.
72. Huang J, Wu Y, Wang M, Jiang J, Zhu Y, Kumar R, et al. The global disease burden of varicella-zoster virus infection from 1990 to 2019. J Med Virol. 2022;94(6):2736-46.
73. Safonova E, Yawn BP, Welte T, Wang C. Risk factors for herpes zoster: should people with asthma or COPD be vaccinated? Respir Res. 2023;24(1):35.
74. Kawai K, Yawn BP, Wollan P, Harpaz R. Increasing Incidence of Herpes Zoster Over a 60-year Period From a Population-based Study. Clin Infect Dis. 2016;63(2):221-6.
75. Cunningham AL, Lal H, Kovac M, Chlibek R, Hwang SJ, Díez-Domingo J, et al. Efficacy of the Herpes Zoster Subunit Vaccine in Adults 70 Years of Age or Older. N Engl J Med. 2016;375(11):1019-32.
76. Boutry C, Hastie A, Diez-Domingo J, Tinoco JC, Yu CJ, Andrews C, et al. The Adjuvanted Recombinant Zoster Vaccine Confers Long-Term Protection Against Herpes Zoster: Interim Results of an Extension Study of the Pivotal Phase 3 Clinical Trials ZOE-50 and ZOE-70. Clin Infect Dis. 2022;74(8):1459-67.
77. Tseng HF, Harpaz R, Luo Y, Hales CM, Sy LS, Tartof SY, et al. Declining Effectiveness of Herpes Zoster Vaccine in Adults Aged ≥60 Years. J Infect Dis. 2016;213(12):1872-5.
78. Current product information of Shingrix® available at www.swissmedicinfo.ch.
79. Chen J, Shin JY, Kim H, Kim JH, Choi A, Cheong HJ, et al. Incidence and Healthcare Burden of Pertussis among Older Adults with and without Pre-Existing Chronic Obstructive Pulmonary Disease or Asthma in South Korea. Copd. 2023;20(1):126-34.
80. Aris E, Harrington L, Bhavsar A, Simeone JC, Ramond A, Papi A, et al. Burden of Pertussis in COPD: A Retrospective Database Study in England. Copd. 2021;18(2):157-69.
81. Domenech de Cellès M, Rohani P. Pertussis vaccines, epidemiology and evolution. Nat Rev Microbiol. 2024.
82. Current product information of Boostrix® available at www.swissmedicinfo.ch.
83. Current product information of Adacel® available at www.swissmedicinfo.ch.
84. Koepke R, Eickhoff JC, Ayele RA, Petit AB, Schauer SL, Hopfensperger DJ, et al. Estimating the effectiveness of tetanus-diphtheria-acellular pertussis vaccine (Tdap) for preventing pertussis: evidence of rapidly waning immunity and difference in effectiveness by Tdap brand. J Infect Dis. 2014;210(6):942-53.
85. Gierke R. Current Epidemiology of Pneumococcal Disease among Adults, United States. Presented at the Advisory Committee on Immunization Practices (ACIP) Meeting on February 29, 2024.
86. Centers for Disease Control and Prevention (CDC). Pneumococcal Disease. https://www.cdc.gov/pneumococcal/hcp/vaccine-recommendations/index.html; last accessed September 2024.
87. de Boer PT, van Werkhoven CH, van Hoek AJ, Knol MJ, Sanders EAM, Wallinga J, et al. Higher-valency pneumococcal conjugate vaccines in older adults, taking into account indirect effects from childhood vaccination: a cost-effectiveness study for the Netherlands. BMC Med. 2024;22(1):69.
88. Platt H, Omole T, Cardona J, Fraser NJ, Mularski RA, Andrews C, et al. Safety, tolerability, and immunogenicity of a 21-valent pneumococcal conjugate vaccine, V116, in healthy adults: phase 1/2, randomised, double-blind, active comparator-controlled, multicentre, US-based trial. Lancet Infect Dis. 2023;23(2):233-46.
89. Wassil J, Sisti M, Fairman J, Davis M, Fierro C, Bennett S, et al. Evaluating the safety, tolerability, and immunogenicity of a 24-valent pneumococcal conjugate vaccine (VAX-24) in healthy adults aged 18 to 64 years: a phase 1/2, double-masked, dose-finding, active-controlled, randomised clinical trial. Lancet Infect Dis. 2024;24(3):308-18.
90. Piralla A, Chen Z, Zaraket H. An update on respiratory syncytial virus. BMC Infect Dis. 2023;23(1):734.
91. Du Y, Yan R, Wu X, Zhang X, Chen C, Jiang D, et al. Global burden and trends of respiratory syncytial virus infection across different age groups from 1990 to 2019: A systematic analysis of the Global Burden of Disease 2019 Study. Int J Infect Dis. 2023;135:70-6.
92. Current product information of Arexvy® (RSVPreF3-Antigen) available at www.swissmedicinfo.ch.
93. Federal Office of Public Health (FOPH). Respiratory syncycial virus (RSV). https://www.bag.admin.ch/bag/en/home/krankheiten/krankheiten-im-ueberblick/rsv.html; last accessed October 2024.
94. Surie D, Yuengling KA, DeCuir J, Zhu Y, Gaglani M, Ginde AA, et al. Disease Severity of Respiratory Syncytial Virus Compared with COVID-19 and Influenza Among Hospitalized Adults Aged ≥60 Years – IVY Network, 20 U.S. States, February 2022-May 2023. MMWR Morb Mortal Wkly Rep. 2023;72(40):1083-8.
95. Wyffels V, Kariburyo F, Gavart S, Fleischhackl R, Yuce H. A Real-World Analysis of Patient Characteristics and Predictors of Hospitalization Among US Medicare Beneficiaries with Respiratory Syncytial Virus Infection. Adv Ther. 2020;37(3):1203-17.
96. Osei-Yeboah R, Johannesen CK, Egeskov-Cavling AM, Chen J, Lehtonen T, Fornes AU, et al. Respiratory Syncytial Virus-Associated Hospitalization in Adults With Comorbidities in 2 European Countries: A Modeling Study. J Infect Dis. 2024;229(Supplement_1):S70-s7.
97. Woodruff RC, Melgar M, Pham H, Sperling LS, Loustalot F, Kirley PD, et al. Acute Cardiac Events in Hospitalized Older Adults With Respiratory Syncytial Virus Infection. JAMA Intern Med. 2024;184(6):602-11.
98. Ramilo O, Rodriguez-Fernandez R, Mejias A. Respiratory Syncytial Virus Infection: Old Challenges and New Approaches. J Infect Dis. 2023;228(1):4-7.
99. Papi A, Ison MG, Langley JM, Lee DG, Leroux-Roels I, Martinon-Torres F, et al. Respiratory Syncytial Virus Prefusion F Protein Vaccine in Older Adults. N Engl J Med. 2023;388(7):595-608.
100. Walsh EE, Pérez Marc G, Zareba AM, Falsey AR, Jiang Q, Patton M, et al. Efficacy and Safety of a Bivalent RSV Prefusion F Vaccine in Older Adults. N Engl J Med. 2023;388(16):1465-77.
101. Wilson E, Goswami J, Baqui AH, Doreski PA, Perez-Marc G, Zaman K, et al. Efficacy and Safety of an mRNA-Based RSV PreF Vaccine in Older Adults. N Engl J Med. 2023;389(24):2233-44.
102. Falsey AR, Hosman T, Bastian AR, Vandenberghe S, Chan EKH, Douoguih M, et al. Long-term efficacy and immunogenicity of Ad26.RSV.preF-RSV preF protein vaccine (CYPRESS): a randomised, double-blind, placebo-controlled, phase 2b study. Lancet Infect Dis. 2024.
103. Ison MG, Papi A, Athan E, Feldman RG, Langley JM, Lee DG, et al. Efficacy and Safety of Respiratory Syncytial Virus (RSV) Prefusion F Protein Vaccine (RSVPreF3 OA) in Older Adults Over 2 RSV Seasons. Clin Infect Dis. 2024;78(6):1732-44.
104. Hause AM, Moro PL, Baggs J, Zhang B, Marquez P, Melgar M, et al. Early Safety Findings Among Persons Aged ≥60 Years Who Received a Respiratory Syncytial Virus Vaccine – United States, May 3, 2023-April 14, 2024. MMWR Morb Mortal Wkly Rep. 2024;73(21):489-94.
105. Moreira AC, Ribeiro AB, Oliveira I, Sá M, Lameirão C, Marques P. Efficacy of anti-RSV vaccination in preventing respiratory syncytial virus disease and severe illness in older adults: a systematic review of randomized controlled trials. Eur Geriatr Med. 2024.
106. Centers for Disease Control and Prevention (CDC). New Respiratory Syncytial Virus (RSV) Vaccines for Adults: General Information and Clinical Guidance. Current Issues in Immunization Webinar, August 30th, 2023.
107. Rademacher J, Therre M, Hinze CA, Buder F, Böhm M, Welte T. Association of respiratory infections and the impact of vaccinations on cardiovascular diseases. Eur J Prev Cardiol. 2024;31(7):877-88.
108. World Health Organisation (WHO). Increasing and sustaining acceptance of and demand for vaccination. https://www.who.int/europe/activities/increasing-and-sustaining-acceptance-of-and-demand-for-vaccination; last accessed October 2024.
PRAXIS
- Vol. 113
- Ausgabe 11-12
- Dezember 2024