- Antibiotic Prophylaxis in Gastrointestinal Endoscopy
Introduction
The need for antibiotic prophylaxis for endoscopic procedures, especially in the gastrointestinal tract, has long been a matter of debate. In recent years, the lack of randomized trials supporting a benefit of antibiotic prophylaxis, the very low incidence of infective endocarditis after endoscopic procedures and the potential adverse reactions of antibiotics have led to a more restricted use of antibiotic prophylaxis.
The aim of this expert opinion statement is to provide an overview of current evidence and to propose a pragmatic approach to the use of antibiotic prophylaxis for endoscopic procedures in areas where clear evidence is lacking.
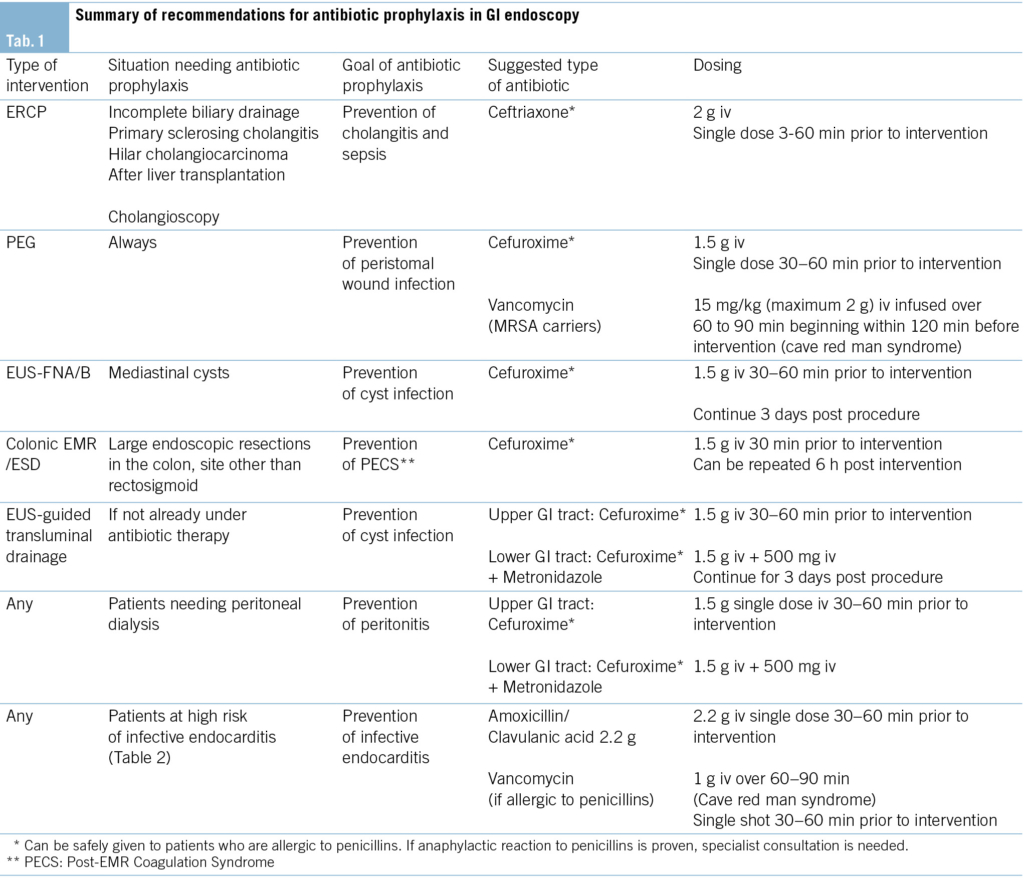
All recommended antibiotic regimens are summarized in Tab. 1.
The GRADE system is used for rating the quality of evidence.
High-quality evidence: recommendation grade A
Evidence comes from one or more well-designed and well-executed randomized controlled trials that yield consistent and directly applicable results. Further research is very unlikely to change our confidence in the estimate of effect.
Moderate-quality evidence: recommendation grade B
Evidence comes from randomized controlled trials with important limitations and a very small number of participants, well-designed controlled trials without randomization, well-designed cohort or case-control studies, and multiple time series with or without intervention. Further research will probably have an important impact on our confidence in the estimate of effect and may change the estimate.
Low-quality evidence: recommendation grade C
Evidence comes from observational studies. Further research is very likely to have an important impact on our confidence in the estimate of effect and will probably change the estimate.
Very-low quality evidence: recommendation grade D
Evidence is conflicting, of poor quality, or lacking, and hence the balance of benefits and harms cannot be determined. Any estimate of effect is very uncertain as evidence is either unavailable or does not permit a conclusion.
Risk of bacteremia
Transient bacteremia is very common during many routine daily activities, such as tooth brushing (up to 68 %), using toothpicks (up to 40 %), or even chewing food (up to 50 %). These numbers are essential to consider when evaluating the incidence of transient bacteremia associated with gastrointestinal procedures.
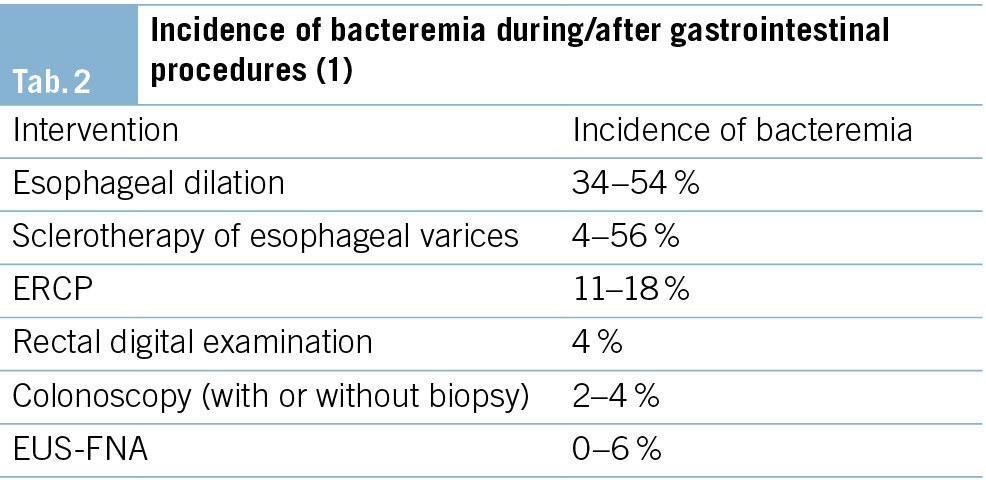
Gastroscopy, flexible sigmoidoscopy and colonoscopy are all considered low-risk procedures for bacteremia and infection, regardless of whether biopsies are taken or polypectomies are performed. Mean rates of bacteremia were estimated to be 1 % in sigmoidoscopy and 4 % in gastroscopy and colonoscopy, whereas esophageal dilation carries the highest risk for bacteremia. (Tab. 2)
Endoscopic procedures with high-risk for bacteremia
ERCP in an obstructed bile duct
The risk for bacteremia increases from 6 % in the absence of biliary obstruction to 18 % with obstruction (2). Therefore, in cases of biliary obstruction the incidence of post-ERCP cholangitis is increased.
Esophageal dilation
Bacteremia during or after esophageal dilation is found in up to 22 % of patients and may be higher in the case of multiple passes and/or in patients with malignant stenosis (3, 4).
Sclerotherapy of varices
The range of reported bacteremia rates is wide, spanning between 4 % and 56 % in different studies, with an average rate around 20 % in most studies (5).
In contrast, rubber band ligation of esophageal varices is not considered a high-risk procedure for bacteremia, with an estimated risk 9 % (6).
Risk of infective endocarditis after endoscopic procedures
Although bacteremia, as outlined above, is a common event during endoscopic procedures, subsequent infective endocarditis (IE) is extremely rare. Despite the constantly rising number of endoscopic procedures performed worldwide, there has been no evidence of an increasing incidence of IE after such procedures.
Furthermore, only limited data exist regarding the impact of antibiotic prophylaxis for dental or surgical procedures on the prevention of IE. Failures of endocarditis prophylaxis, despite the correct administration of antibiotic prophylaxis, are well recognized (7). For these reasons, antibiotic prophylaxis is generally not recommended to reduce the incidence of infective endocarditis in GI endoscopic procedures.
Patients at high risk of infective endocarditis
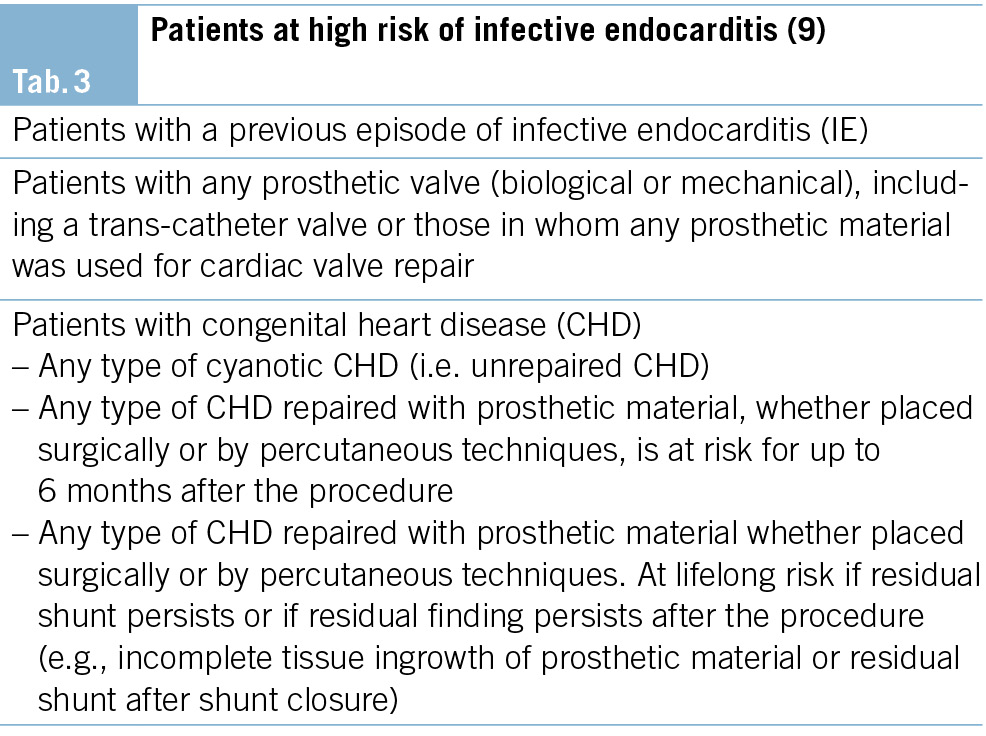
In the recently published guideline of the European Society of Cardiology, there is a novel recommendation for patients at high risk of infective endocarditis (Tab. 3), stating that “antibiotic prophylaxis may be considered for high-risk patients undergoing an invasive diagnostic or therapeutic procedure in the gastrointestinal and genitourinary tract, skin or musculoskeletal system” (8).
There is no evidence supporting the use of antimicrobial prophylaxis for cardiac transplant recipients who develop cardiac valvulopathy. The indication should be discussed on a case-by-case basis. Patients should contact their transplant specialist to evaluate the indication prior to an elective intervention.
To prevent infective endocarditis, antibiotic prophylaxis may be considered in high-risk patients undergoing an invasive diagnostic or therapeutic procedure in the GI tract (Tab. 2) (low quality evidence).
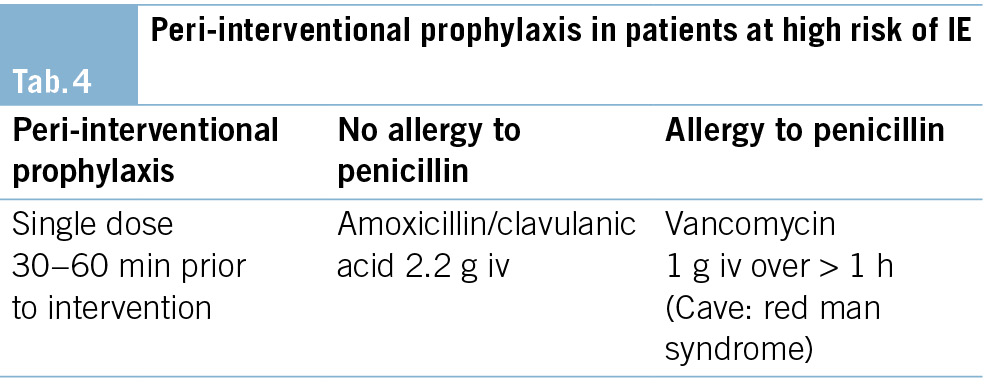
In patients at high risk of IE, the following antibiotic regimen is recommended (9). (Tab. 4)
Endoscopic procedures due to infective disorders in high-risk patients of infective endocarditis
In the case of an established infection likely caused by enterococci, an empiric antibiotic regimen with anti-enterococcal activity should be used. These patients, if already receiving antibiotic therapy, do not need additional antibiotic prophylaxis when undergoing an endoscopic procedure (Tab. 3).
Specific endoscopic procedures: When to use antibiotic prophylaxis
Routine upper endoscopy and colonoscopy
Antibiotic prophylaxis is not required in routine gastrointestinal endoscopy including biopsies and polypectomy, even if high-risk procedures are performed during routine endoscopy (Tab. 2). Exceptions may include patients with severe neutropenia (< 500 cells/mm3) and advanced hematologic malignancies (35).
Colorectal endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD)
EMR and ESD are frequently performed to treat benign and early malignant colorectal lesions. However, infections following EMR and ESD are extremely rare.
Routine use of antibiotic prophylaxis is therefore not recommended.
Post-EMR/ESD Coagulation Syndrome (PECS)
In the context of large colorectal endoscopic resections, a novel complication known as “post-EMR/ESD coagulation syndrome” (PECS) has been recognized. The syndrome is characterized by pain, local peritonitis, fever and elevation of inflammatory markers. It occurs in up to 40 % of patients undergoing a large endoscopic resection.
Large lesions (>30 mm) and localization outside the rectosigmoid colon are independent risk factors for the development of PECS. Limited data are available on the effect of antibiotic prophylaxis in such patients. In one randomized controlled trial of 409 patients (randomized to either cefuroxime 1.5 g iv half an hour before and 6 hours after the intervention or placebo), the rate of adverse events in the antibiotic group was significantly lower than in the control group: abdominal pain (2.8 % vs 14.9 %, p < 0.01), diarrhea (2.0 % vs 9.3 %, p < 0.05), and fever (0.9 % vs 8.4 %, p < 0.05), respectively. The levels of inflammatory markers were also significantly lower in the antibiotic group compared with those in the control group (11). Therefore, antibiotic prophylaxis can be applied to reduce the risk of PECS in patients with large endoscopic resections above the rectosigmoid colon.
To prevent PECS, antibiotic prophylaxis can be considered in patients with large colonic endoscopic resections above the sigmoid colon (low-quality evidence).
Endoscopic retrograde cholangio-pancreatography (ERCP)
Cholangitis is the most common infectious adverse event in ERCP. Others are cholecystitis, duodenoscope-related transmission of infections, and endocarditis (12).
The role of antibiotic prophylaxis in reducing the risk of post-ERCP cholangitis has been evaluated in several studies. The most recent a meta-analysis from 2010 (9 RCTs with 1573 patients) (13) found a lower risk of post-ERCP cholangitis. In the subgroup of patients who were drained after the first ERCP, there was no benefit from antibiotic prophylaxis.
Subsequent studies did not find a benefit of antibiotic prophylaxis except in patients with biliary obstruction. A very recent randomized trial of 378 patients found a significantly reduced rate of infectious complications, especially cholangitis (2.8 % vs 9.8 % p = 0.007) in patients with biliary obstruction who received antibiotic prophylaxis (14).
Antibiotic prophylaxis is not recommended if cholangitis is absent, if biliary drainage is likely to be successful, and in patients undergoing ERCP for reasons other than biliary obstruction (high-quality evidence).
Malignant hilar obstruction and PSC
Especially in patients with malignant hilar obstruction and primary sclerosing cholangitis, the risk of unsuccessful drainage is higher, which raises the risk of complicating cholangitis (15).
Peroral cholangioscopy
Several studies have found that peroral cholangioscopy is associated with a high risk of bacteremia and cholangitis, regardless of the indication for the intervention. For this reason, all patients undergoing peroral cholangioscopy should receive antibiotic prophylaxis (16).
To prevent post-ERCP cholangitis and/or sepsis, antibiotic prophylaxis is recommended for patients with biliary obstruction, PSC, and in patients undergoing cholangioscopy (moderate-quality evidence).
In the case of failed biliary drainage, antibiotic therapy may be continued for 3–5 days (17).
Endosonographic-guided tissue acquisition
EUS-guided puncture/biopsy is an important, minimally invasive technique for obtaining tissue diagnoses from a variety of pancreatic, intraabdominal, retroperitoneal, or mediastinal lesions in close proximity to the gastrointestinal tract. The major complications associated with EUS-FNA include hemorrhage, perforation, infection, and organ-specific complications, such as acute pancreatitis following puncture of pancreatic lesions. A previous systematic review of complications and deaths associated with EUS-FNA (51 reports, 10 941 patients) revealed an overall complication rate of 0.98 %. The risk of infection was very low, at 0.05 % (18). The risk of infection depends on the type of lesion being sampled. Data suggest that solid lesions carry a very low risk for infection (0.01 %–2 %). The risk of infection following EUS-FNA of cystic lesions is less clear, with reported rates of infection ranging from < 1 % to 14 % (19).
Pancreatic lesions
Solid lesions of the pancreas
EUS-FNA and EUS-FNB carry a low risk of bacteremia and sepsis, especially in solid lesions. In two large series involving 627 patients undergoing EUS-FNA for a variety of solid lesions of the pancreas, sepsis developed in only 3 patients.
Antibiotic prophylaxis is not recommended before EUS-FNA/B of solid lesions of the pancreas (moderate-quality evidence).
Cystic lesions of the pancreas
It has been a long-standing practice to administer antibiotic prophylaxis in patients undergoing EUS-FNA of cystic pancreatic lesions, since older data suggest that this intervention is associated with higher rates of infection and antibiotic prophylaxis appeared to be efficient in such patients.
However, newer data do not support this practice and the role of antibiotic prophylaxis has been questioned. A meta-analysis from 2020 (six studies, including one randomized controlled trial and five retrospective studies, with 1706 patients) evaluated the efficacy of antibiotic prophylaxis prior to EUS-FNA of cystic pancreatic lesions. Overall, 8 infectious events were observed in the antibiotic group (0.77 %), and 12 events in the control group (1.7 %), (odds ratio (OR) 0.65, 95 % confidence interval (95 % CI) 0.24–1.78; p = 0.40). No difference was observed between the two study groups in terms of either severe infection (OR 0.88, 95 % CI 0.13–5.82; p = 0.89) or overall adverse event rate (OR 1.09, 95 % CI 0.73–1.65; p = 0.67). These findings suggest prophylactic antibiotics do not substantially reduce the risk of infections after EUS-FNA of cystic lesions of the pancreas (20).
One randomized trial from Spain compared the effect of antibiotic prophylaxis versus placebo in patients undergoing EUS-FNA of cystic lesions of the pancreas. More than 200 patients were randomly assigned to prophylaxis with ciprofloxacin (n = 112) or saline solution (n = 114, placebo). The only case of FNA-related infection (0.44 %) occurred in a patient in the placebo group (0.87 %). Prevention of infection was not inferior in the control group, and there were no differences between groups regarding the occurrence of post-interventional fever or other adverse events (21).
We suggest that antibiotic prophylaxis can be retained in patients undergoing EUS-FNA of cystic lesions of the pancreas and may be reserved for special situations (moderate-quality evidence)
Rectal and perirectal lesions
EUS-guided transrectal tissue acquisition is a safe technique to obtain tissue diagnosis of solid perirectal lesions. The infection rate after such interventions is very low.
In a prospective study, 100 patients underwent a total of 471 fine needle aspirations of rectal or perirectal lesions. Blood cultures were taken in all patients before and after the intervention. Of these, cultures were positive in 6 patients and 4 patients had contamination. Two patients developed bacteremia with either Bacteroides fragilis or Gemella morbillorum. No signs or symptoms of infection developed in any patient.
Therefore, EUS-FNA of solid lesions in the lower GI tract can be considered a low-risk procedure for infection and does not warrant antibiotic prophylaxis (22).
Antibiotic prophylaxis is not recommended in patients undergoing EUS-FNA of solid perirectal lesions (moderate-quality evidence).
Mediastinal lesions
EUS-guided transesophageal puncture is a safe technique that allows tissue acquisition for diagnosis of undetermined mediastinal lesions. Special caution is required with cystic lesions, as post-interventional infection of such lesions may be life threatening.
Solid mediastinal lesions
The complication rate is low when EUS-FNA is performed in mediastinal lymph nodes and solid tumors. A systematic review and meta-analysis of EUS-FNA in mediastinal lymph node lesions in patients with non-small cell lung cancer (18 reports, 1201 patients) (23) observed only minor complications, such as sore throat and fever, in a minority of patients (0.8 %). No infectious complications, such as mediastinitis, have been reported.
Antibiotic prophylaxis is not recommended for EUS-FNA of solid mediastinal lesions (moderate-quality evidence).
Cystic mediastinal lesions
Several case reports and case series describe infections following EUS-FNA of cystic mediastinal lesions of different etiologies, making it a high-risk procedure for infection. Some of these infections occurred despite the use of prophylactic antibiotics (24), thus the indication for EUS-FNA of such lesions should be chosen wisely. Needles with smaller diameters (25G/22G) may have a lower risk of infection and larger needles should be avoided (25). In cases of EUS-FNA of a cystic mediastinal lesion, a second-generation cephalosporin should be used.
To prevent cyst infection and/or mediastinitis antibiotic prophylaxis is recommended prior to and for about 3 days following EUS-FNA of cystic mediastinal lesions (low-quality evidence)
EUS-guided transluminal interventions
The most common reason for EUS-guided transluminal drainage of fluid collections is infection. As a result, these patients are usually receiving antibiotic therapy. If drainage is performed for indications other than infection (e.g., obstruction, pain, other) antibiotic prophylaxis is recommended: cefuroxime can be used in the upper GI tract, cefuroxime plus metronidazole in the lower GI tract.
To prevent cyst infection and/or sepsis antibiotic prophylaxis is recommended in patients undergoing EUS-guided transluminal interventions (low-quality evidence).
Percutaneous endoscopic gastrostomy (PEG)
Peristomal wound infection is the most common complication of PEG placement. A Cochrane review of 12 randomized trials including 1271 patients (OR 0.36, 95 % CI 0.26 – 0.50) (26) found that antibiotic prophylaxis is effective in preventing this complication, with an NNT of 5–10 to prevent one wound infection. First and second generation cephalosporins (cefazolin, cefuroxime) can also be safely given to patients who are allergic to penicillin (27). They should be avoided in patients who have had a proven anaphylactic reaction to penicillin or angioedema. Patients who are already on broad-spectrum antibiotic therapy do not need additional antibiotics.
To prevent peristomal wound infection antibiotic prophylaxis is recommended in patients undergoing PEG tube placement (high quality evidence).
Methicillin-resistant Staphylococcus aureus (MRSA)
MRSA is likely a negligible problem in Switzerland. Only about 4 % of the Swiss population are MRSA carriers. In contrast, the carrier rate among patients in health care facilities is significantly higher, particularly in long-term care facilities that care for patients requiring feeding tubes.
In patients with nasopharyngeal colonization by MRSA, a significant proportion of peristomal wound infections are MRSA-related. In these situations, MRSA decolonization, if feasible in the clinical context, can reduce the risk of MRSA-related wound infections (28).
If decolonization is not possible, vancomycin is effective in preventing wound infections in patients undergoing PEG placement, as confirmed by two small trials (29, 30).
Endoscopy in patients with liver cirrhosis
Compensated cirrhosis
For patients with compensated liver cirrhosis, the same standards for antibiotic prophylaxis apply as for non-cirrhotic patients.
Antibiotic prophylaxis is not recommended in patients with compensated liver cirrhosis undergoing endoscopy (moderate-quality evidence)
Cirrhosis with ascites
Studies supporting the use of prophylactic antibiotics in patients with liver cirrhosis and ascites are lacking. As a result, antibiotic prophylaxis is generally not recommended.
In contrast to patients with active variceal bleeding, routine antibiotic prophylaxis for patients undergoing elective rubber band ligation of esophageal varices is currently not recommended (moderate quality evidence).
Cirrhotic patients with gastrointestinal bleeding
Patients with liver cirrhosis presenting with acute gastrointestinal bleeding are at high risk of bacterial infection, especially bacterial peritonitis and respiratory tract infections, which occur in about 20 % of these patients. Bacterial infections lead to a higher risk of re-bleeding and an increased overall mortality rate.
A Cochrane analysis of 12 randomized trials including over 1200 patients with gastrointestinal bleeding showed that antibiotic therapy is associated with lower overall mortality (including lower mortality from bacterial infections, lower rates of rebleeding and shorter hospital stay) (31). Antibiotic therapy should be initiated at admission for cirrhotic patients presenting with GI bleeding. Antibiotic therapy should be continued for 7 days. Intravenous ceftriaxone is superior to norfloxacin (32) in the prevention of infection in variceal and non-variceal bleeding in cirrhotic patients.
To prevent infection and rebleeding, antibiotic prophylaxis is recommended in cirrhotic patients presenting with GI bleeding. Treatment should be continued for 7 days. (high quality evidence)
Endoscopy in special situations
Ventriculoperitoneal or lumboperitoneal shunts
There are no data on antibiotic prophylaxis for endoscopy in patients with ventriculoperitoneal or lumboperitoneal shunts. By analogy with abdominal surgery, antibiotic prophylaxis is not recommended because, even in non-sterile abdominal surgery, infectious shunt complications are rare (33).
Antibiotic prophylaxis is not recommended in patients with ventriculoperitoneal or lumboperitoneal shunts undergoing endoscopy (moderate quality evidence).
Peritoneal dialysis
Antibiotic prophylaxis is recommended in peritoneal dialysis patients undergoing colonoscopy (especially with polypectomy) due to the risk of bacterial translocation,
One retrospective study showed that the risk of peritonitis after colonoscopy without antibiotic prophylaxis was 6.3 % (34). Before endoscopy, ascites should be completely drained (35, 36).
To prevent peritonitis, antibiotic prophylaxis is recommended in patients undergoing peritoneal dialysis and endoscopy (moderate quality evidence)
Orthopedic prosthesis
Infection of prosthetic joints related to endoscopic procedures in the GI tract is extremely rare. Given the low incidence of joint infections following endoscopy, prophylactic antibiotics are not currently recommended by the American Society of Gastroenterologists or the American Society of Colon and Rectal Surgeons (37, 38).
In addition, prophylactic antibiotics are no longer recommended in the antibiotic prophylaxis guidelines from the American Academy of Orthopedic Surgeons (AAOS) (39).
Antibiotic prophylaxis is not recommended in patients with orthopedic prostheses undergoing endoscopy (moderate quality evidence).
Endoscopy in patients with vascular grafts
The administration of antibiotic prophylaxis is not recommended in patients with synthetic vascular grafts and other non-valvular cardiovascular devices, such as pacemakers, defibrillators, coronary artery stents, peripheral vascular stents, and vena cava filters.
According to the American Heart Association (AHA), there is no evidence that microorganisms associated with GI endoscopic procedures cause infection of non-valvular cardiovascular devices, including synthetic vascular grafts, at any time after implantation. Thus, antimicrobial prophylaxis is not recommended for any endoscopic procedure in patients with cardiovascular implantable electronic devices (40, 41).
Antibiotic prophylaxis is not recommended in patients with vascular grafts and non-valvular cardiovascular devices (moderate-quality evidence).
Endoscopy in immunocompromised patients
In severe neutropenia (absolute neutrophil count < 500 cells/mL) and in patients with advanced hematologic malignancies, there is an increased risk of bacteremia and sepsis after GI endoscopy (42). Although not extensively studied, it seems reasonable, especially in patients undergoing endoscopic procedures that are associated with a high risk of bacteremia, to administer antibiotic prophylaxis.
To prevent bacteremia and sepsis, antibiotic prophylaxis is recommended in patients with severe neutropenia and hematologic malignancies undergoing endoscopy (low-quality evidence).
In immunocompromised patients (i.e., organ transplant recipients, patients with HIV) who have normal neutrophil counts, routine administration of prophylactic antibiotics is not recommended. It is unclear whether patients with other causes of immunosuppression (including those on high doses of glucocorticoids) benefit from antibiotic prophylaxis.
Abbreviations
CHD Congenital Heart Disease
EMR Endoscopic Mucosal Resection
ERCP Endoscopic Retrograde Cholangio-Pancreatography
ESD Endoscopic Submucosal Dissection
EUS-FNA Endoscopic Ultrasound guided Fine Needle Aspiration
EUS-FNB Endoscopic Ultrasound guided Fine Needle Biopsy
GI Gastrointestinal
IE Infective Endocarditis
MRSA Methicillin Resistant Staphylococcus Aureus
NNT Number Needed to Treat
PECS Post EMR / ESD Coagulation Syndrome
PEG Percutaneous Endoscopic Gastrostomy
PSC Primary Sclerosing Cholangitis
RCT Randomized Controlled Trial
History
Manuscript received: 03.03.2025
Manuscript accepted: 11.03.2025
Michael Manz* 1, Stefan Kuster 2, Matthias Greutmann 3, Remus Frei* 2
*contributed equally to this manuscript
Stefan Kuster: Swiss Society for Infectious Diseases
Matthias Greutmann: Swiss expert group on Infective Endocarditis Prevention
Reviewed by: Patrick Aepli 4, Martin Geyer 5, Sebastien Godat 6, Gianluca Lollo 7, Stefan Seewald 8, Frans Olivier The 9, Reiner Wiest 10
Reviewed and approved by SGG council members (2024): Alain Vonlaufen 11, Tobias Ehmann 12, Jan Borovicka 2, David Semela 2, Lukas Degen 13, Stephan Brand 2, Florian Riniker 14, Sophie Buyse 15, Daniele Riva 16, Kaspar Truninger 17, Ellen Utzinger 18
1 Clarunis Universitäres Bauchzentrum Basel und Gastroenterologie Praxis Basel, 2 HOCH, Cantonal Hospital St. Gallen, 3 Universitätsspital Zürich, 4 Luzerner Kantonsspital, 5 Gastroenterologie Praxis Wettingen, 6 CHUV Lausanne, 7 Division of Gastroenterology & Hepatology, Ente Ospedaliero Cantonale Bellinzona, 8 Gastrozentrum Hirslanden, Zürich, 9 Stadtspital Waid, Zürich, 10 Inselspital Bern, 11 Clinique Générale-Beaulieu, Genève, 12 Spital Zofingen, 13 Clarunis Universitäres Bauchzentrum Basel, 14 Gastroenterologie Aarau AG, 15 Centre Yverdonnois de Gastroentérologie et Endoscopies, 16 Gastrocentro Lugano, 17 Klinik für Gastroenterologie und Hepatologie, Universitätsspital Zürich, 18 Fachärztezentrum Glatt, Kantonsspital Winterthur
Leitender Arzt
HOCH, Cantonal Hospital St.Gallen
Clinic of Gastroenterology / Hepatology
Rorschacher Strasse 95, 9007 St.Gallen
remus.frei@h-och.ch
The authors did not declare any conflicts of interest in relation to this article.
1. Allison MC, Sandoe JAT, Tighe R, Simpson IA, Hall RJ. Antibiotic prophylaxis in gastrointestinal endoscopy. Gut 2009;6:869-80.
2. Nelson DB. Infectious disease complications of GI endoscopy: Part I, endogenous infections. Gastrointest Endosc. 2003;4:546-56.
3. Zuccaro G Jr, Richter JE, Rice TW et al. Viridans streptococcal bacteremia after esophageal stricture dilation. Gastrointest Endosc. 1998;6:568-573.
4. Nelson DB, Sanderson SJ, Azar MM. Bacteremia with esophageal dilation. Gastrointest Endosc. 1998 6:563-567.
5. Jia Y, Dwivedi A, Elhanafi S et al.: Low risk of bacteremia after endoscopic variceal therapy for esophageal varices: a systematic review and meta-analysis. Endosc Int Open 2015;5:E409-417.
6. Berner JS, Gaing AA, Sharma R, Almenoff PL, Muhlfelder T, Korsten MA. Sequelae after esophageal variceal ligation and sclerotherapy: a prospective randomized study. Am J Gastroenterol. 1994;6:852-858.
7. Durack DT, Kaplan EL, Bisno AL. Apparent failures of endocarditis prophylaxis: analysis of 52 cases submitted to a national registry. JAMA 1983;250:2318–2322
8. Delgado V, Marsan NA, de Waha S et al. 2023 ESC Guidelines for the management of endocarditis 2023. Eur Heart J. 2023;44:3948–4042.
9. Sendi P, Hasse B, Frank M et al. Infective endocarditis: prevention and antibiotic prophylaxis, the swiss expert group on Infective Endocarditis Prevention, Swiss medical weekly 2021;151:w20473.
10. Jung D, Youn YH, Jahng J, Kim JH, Park H et al: Risk of electrocoagulation syndrome after endoscopic submucosal dissection in the colon and rectum. Endoscopy 2013;45:714-717.
11. Zhang QS, Han B, Xu JH, Gao P, Shen YC. Antimicrobial prophylaxis in patients with colorectal lesions undergoing endoscopic resection. 2015;21:4715-4721.
12. Shinin Merchan MF, de Moura DHT, de Oliviera GHP et al: Antibiotic prophylaxis to prevent complications in endoscopic retrograde cholangiopancreatography: A systematic review and meta-analysis of randomized controlled trials. World J Gastrointest Endosc 2022;14:718-730.
13. Brand M, Bizos D, O’Farrell PJR. Antibiotic prophylaxis for patients undergoing elective endoscopic retrograde cholangiopancreatography. Cochrane Database Syst Rev. 2010.
14. Leem G, Sung MJ, Park JH et al: Randomized Trial of Prophylactic Antibiotics for Endoscopic Retrograde Cholangiopancreatography in Patients With Biliary Obstruction. Am J Gastroenterology 2024; 119:183-190.
15. Dumonceau JM, Kapral C, Aabacken L et al: ERCP-related adverse events: European Society of Gastrointestinal Endoscopy (ESGE) Guideline: Endoscopy 2020;52:127-149.
16. Othman MO, Guerrero R, Elhanafi S et al: A prospective study of the risk of bacteremia in directed cholangioscopic examination of the common bile duct. Gastrointest Endosc 2016;83:151-157.
17. Byl B, Devière J, Struelens MJ, et al. Antibiotic prophylaxis for infectious complications after therapeutic endoscopic retrograde cholangiopancreatography: a randomized, double-blind, placebo-controlled study. Clin Infect Dis 1995;20:1236-1240.
18. Wang, KX, Ben QW, Jin ZD et al: Assessment of morbidity and mortality associated with EUS-guided FNA: A systematic review. Gastrointest Endosc. 2011;73(2):283-290.
19. Guarner-Argente C, Sha P, Buchner A, Ahmad NA, Kochman ML, Ginsberg GG. Use of antimicrobials for EUS-guided FNA of pancreatic cysts: a retrospective, comparative analysis. Gastrointest Endosc. 2011;74:81-86.
20. Facciorusso A, Mohan BP, Tacelli M et al: Use of antibiotic prophylaxis is not needed for endoscopic ultrasound-guided fine-needle aspiration of pancreatic cysts: A meta-analysis. Expert Rev Gastroenterol Hepatol. 2020;14:999-1005.
21. Colán-Hernández J, Sendino O, Loras C et al. Antibiotic Prophylaxis Is Not Required for Endoscopic Ultrasonography-Guided Fine-Needle Aspiration of Pancreatic Cystic Lesions, Based on a Randomized Trial. Gastroenterology. 2020;158:1642-1649.
22. Levy MJ, Norton ID, Clain JE et al. Prospective study of bacteriemia and complications with EUS FNA of rectal and perirectal lesions. Clin Gastroenterol Hepatol. 2007;5:684-689.
23. Micames CG, McCrory DC Pavey DA, Jowell PS, Gress FG. Endoscopic ultrasound-guided fine-needle aspiration for non-small cell lung cancer staging: A systematic review and meta-analysis. Chest 2007;131:539-548.
24. Diehl DL, Cheruvattath R, Facktor MA, Go BD. Infection after endoscopic ultrasound-guided aspiration of mediastinal cysts. Interact Cardiovasc Thorac Surg. 2010;10:338-340.
25. Fazel A, Moezardalan K, Varadarajulu S, Draganov P, Eloubeidi MA et al. The utility and the safety of EUS-guided FNA in the evaluation of duplication cysts. Gastrointest Endosc. 2005;62:575-580.
26. Lipp A, Lousardi G. Systemic antimicrobial prophylaxis for percutaneous endoscopic gastrostomy. Cochrane Database Syst Rev. 2013;2013:CD005571.
27. Apter AJ, Kinman JL, Bilker WB et al: Is there cross-reactivity between penicillins and cephalosporins? Am J Med 2006;119:354.
28. Horiuchi A, Nakayama Y, Kajiyama M, Fujii H, Tanaka N. Nasopharyngeal decolonization of methicillin-resistant Staphylococcus aureus can reduce PEG peristomal wound infection. Am J Gastroenterol 2006;101:274-277.
29. Rao GG, Osman M, Johnson L, Ramsey D, Jones S, Fidler H. Prevention of percutaneous endoscopic gastrostomy site infections caused by methicillin-resistant Staphylococcus aureus. J Hosp Infect 2004;58:81-83.
30. Thomas S, Cantrill S, Waghorn DJ, McIntyre A. The role of screening and antibiotic prophylaxis in the prevention of percutaneous gastrostomy site infection caused by methicillin-resistant Staphylococcus aureus. Aliment Pharmacol Ther.2007;25:593-597.
31. Chavez-Tapia NC, Barrientos-Gutierrez T, Tellez-Avila F et al: Meta-analysis: antibiotic prophylaxis for cirrhotic patients with upper gastrointestinal bleeding – an updated Cochrane review. Aliment Pharmacol Ther.2011;34:509-518.
32. Lee S, Saxinger L, Ma M et al. Bacterial infection in acute variceal bleeding. United European Gastroenterol J. 2017;5:1090-1099.
33. Goel A, Craven C, Matloob S, Thompson S, Watkins L, Toma A. CSF-diverting shunts: Implications for abdominal and pelvic surgeons; a review and pragmatic overview. Ann Med Surg (Lond). 2019;48:100-104.
34. Yip T, Tse KC, Lam MF et al. Risks and outcomes of peritonitis after flexible colonoscopy in CAPD patients. Perit Dial Int 2007;27:560-564.
35. Li PKT, Chov KM, Cho Y et al. ISPD peritonitis guideline recommendations: 2022 update on prevention and treatment. Perit Dial Int.2022;42:110-153.
36. Wu HH, Li IJ, Weng CH et al. Prophylactic antibiotics for endoscopy-associated peritonitis in peritoneal dialysis patients. PLoS One.2013;8 :e71532.
37. Oliver G, Lowry A, Hicks T et al. Practice parameters for antibiotic prophylaxis–supporting documentation. The Standards Task Force. The American Society of Colon and Rectal Surgeons. Dis Colon Rectum.2000;43:1194-1200.
38. ASGE Standards of Practice Committee, Khashab MA, Chithadi KV, Acosta RD et al. Antibiotic prophylaxis for GI endoscopy. Gastrointest Endosc 2015 ;81:81-89.
39. Watters 3rd W, Rethman MP, Hanson NB et al. Prevention of orthopaedic implant infection in patients undergoing dental procedures. J Am Acad Orthop Surg. 2013;21:180-189.
40. Baddour LM, Bettmann MA, Bolger AF et al. Nonvalvular cardiovascular device-related infections. Circulation. 2003;108:2015-2031.
41. Baddour LM, Epstein et al: A summary of the update on cardiovascular implantable electronic device infections and their management: a scientific statement from the American Heart Association. J Am Dent Assoc. 2011;142:159-165.
42. Bianco JA, Pepe MS, Higano C, Applebaum FR, McDonals GB, Singer JW. Prevalence of clinically relevant bacteremia after upper gastrointestinal endoscopy in bone marrow transplant recipients. Am J Med.1990;89:134-136.