- What’s new in cervical cancer in 2024?
Trotz der jüngsten Fortschritte bei Immunisierungs- und Screening-Programmen gibt es bei der Behandlung von fortgeschrittenem und metastasiertem Gebärmutterhalskrebs noch immer ungedeckte Bedürfnisse. Die Einführung der Immuntherapie sowie von Antikörper-Wirkstoff-Konjugaten (ADCs) stellen vielversprechende Optionen dar. Ziel dieser Publikation ist es, die neuesten Erkenntnisse auf diesem Gebiet zusammenzufassen und einen Ausblick auf die künftigen Behandlungsstandards zu geben.
Despite recent advances in immunization and screening programs, there are unmet needs regarding the treatment of advanced and metastatic cervical cancer. The introduction of immunotherapy as well as antibody-drug-conjugates (ADCs) represent promising options. The aim of this publication is to summarize the recent evidence in this field, as well as to provide a perspective on the future standards of care.
Keywords: cervical cancer, Immunotherapy, ADCs.
Introduction
Despite the introduction of human papilloma virus immunization campaigns and enhanced screening procedures (1), cervical cancer remains a leading cause of death worldwide, particularly in low-middle income countries (2). The current cure rate in locally advanced cervical cancer (stages IB2 to IVA) is approximately 70 %. Systemic therapy for advanced disease yields limited results, and thus improved treatment strategies are eagerly awaited in this disease setting.
Treatment of locally advanced disease
The current standard approach is concurrent chemoradiotherapy (CRT) followed by brachytherapy. Two multi-center observational studies in patients with high-risk LAC) receiving chemoradiotherapy (CRT) with magnetic resonance imaging (MRI)-guided adaptive showed long-term benefits (3, 4). Although advances in radiotherapy delivery techniques have indeed improved disease control rates, many patients will nevertheless experience recurrence or progression within 5 years.
OUTBACK (5) is a multicenter, open-label phase 3 trial that evaluated whether the addition of adjuvant chemotherapy after the standard treatment could improve overall survival. 926 patients were randomized to either the standard of care or sequential CRT followed by four cycles of carboplatin and paclitaxel. The trial failed to meet its primary endpoint of overall survival improvement. Moreover, more frequent serious adverse events occurred in the experimental arm.
At the ESMO 2023 meeting, unpublished results of the phase III INTERLACE trial (6) were presented. Briefly, 500 patients were randomly assigned to receive either CRT alone or a sequence of induction chemotherapy over a 6-week period with weekly carboplatin and paclitaxel, then followed by CRT. With a median follow-up of 64 months, a significant progression free survival (PFS) improvement in the induction arm was reported (HR 0.65, 95 % CI 0.46-0.91). The 5-year overall survival (OS) rates also favored the induction arm, being 80 % for the experimental and 72 % for the standard arm (HR 0.61, 95 % CI 0.40-0.91).
Although these data would imply that the addition of induction chemotherapy should become the new standard, some observations are warranted. Firstly, the INTERLACE study enrolled patients over a 10-year period, which means that the radiotherapy delivered in the majority of the study population predates current intensity-modulated techniques, an observation that is also consistent with the fact that the standard arm underperformed as compared to current evidence. In order to put the study findings into context, this approach might prove valuable within a community-based setting as a bridge between initial diagnosis and CRT initiation. However, it is important to emphasize that high quality CRT still remains the core treatment for locally advanced setting, and that excessive delays after the specified induction phase are not advisable.
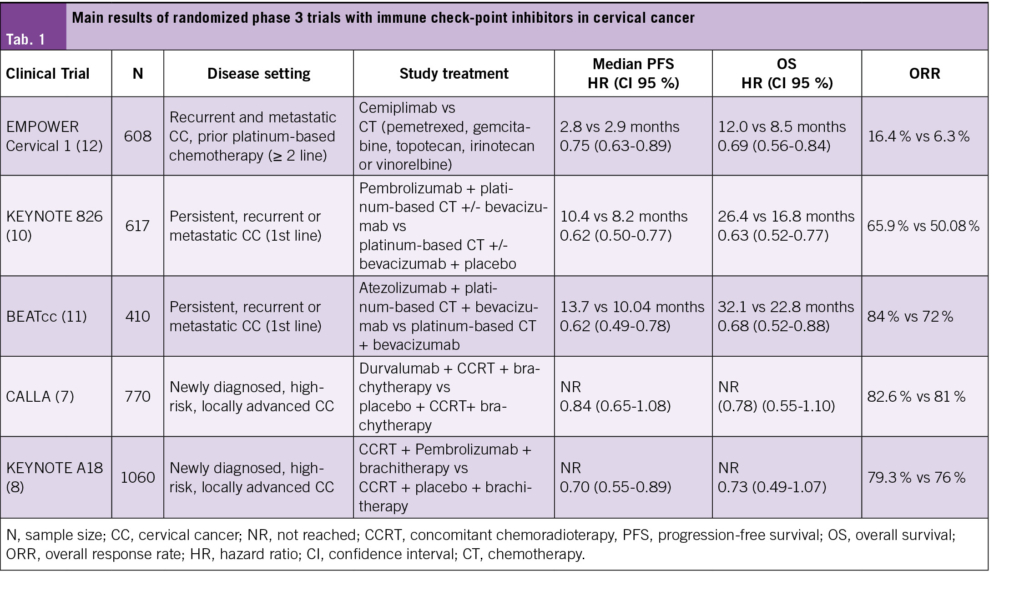
In view of the fact that immunotherapy has proven to be effective in the advanced setting, the phase 3 CALLA trial (7) assessed the benefit of adding monthly durvalumab to standard chemo-radiotherapy, followed by immunotherapy maintenance. The primary endpoint was progression free survival, with key secondary endpoints including overall survival and toxicity. With a median follow-up of 18·5 months, the trial failed to meet its primary endpoint (PFS HR 0·84, 95 % CI 0·65–1·08).
In line with this research question, the recently published phase III KEYNOTE-A18 (8) study also evaluated the efficacy and safety of adding pembrolizumab to concurrent chemoradiotherapy within a comparable population to CALLA, with 1060 patients randomized to receive pembrolizumab plus chemoradiotherapy compared to placebo plus chemoradiotherapy. At the first pre-specified data cutoff and with a median follow-up of 18 months, the experimental arm showed a statistically significant 30 % improvement in PFS (HR 0.70 [95 % CI, 0.55-0.89)). Median PFS was not reached in either group. Overall survival data remain currently immature, but a trend towards and improvement was suggested, as reported during the ESMO 2023 annual conference. A recent press-released confirms that a significant benefit in OS has been achieved while final data are awaited.
These contrasting study results highlight the need to identify better biological and clinical markers to improve patients’ selection with regards to immunotherapy. The CALLA study was overall a negative trial, regardless of the PD-L1 Tumor Area Positivity (TAP) score. Nevertheless, a post-hoc supplementary analysis of TAP PD-L1 cut-offs (7) suggested a PFS benefit in the durvalumab group starting from a TAP score of 20 %. On the other hand, KEYNOTE-A18 analyzed PD-L1 by using the more widely applied Combined Positive Score (CPS). It should also be noted that in KEYNOTE-A18 (8), PD-L1 expression was not predictive of response to pembrolizumab.
Treatment of persistent, recurrent or metastatic disease
Incorporation of immunotherapy into the standard of care
The established first-line therapy in this setting consists of a platinum doublet backbone in combination with bevacizumab, when clinically feasible (9).
KEYNOTE-826 (10) evaluated the efficacy and safety of pembrolizumab in combination with platinum-based chemotherapy with optional bevacizumab in this disease setting. With a median follow-up of 39 months, the addition of pembrolizumab showed meaningful improvements in overall and progression-free survival as compared to the standard of care backbone. Even though the greatest magnitude of benefit in OS was observed among the cohort with a CPS ≥1 %, with a median OS 28.6 months versus 16.5 months (HR 0.60 [95 % CI, 0.49 to 0.74]), it is worth noting that the improvement in OS was maintained in a post-hoc analysis among the overall study population, with a median OS of 26.4 months versus 16.8 months (HR, 0.63 [95 % CI, 0.52 to 0.77]). The safety profile of the experimental arm was manageable, with a similar incidence of grade ≥3 adverse events among arms and less than 5 % discontinuation rate due to immune-related adverse events. In line with the previous trial, no increase in toxicity was observed among the population receiving concomitant bevacizumab.
BEATcc (11) is a randomized, open-label phase 3 trial which enrolled 410 patients to receive at least six cycles of platinum-based chemotherapy plus mandatory bevacizumab with or without atezolizumab, irrespective of their PD-L1 status. With a median follow-up of 33 months, the trial favored the experimental as the dual primary objectives progression-free survival (HR 0·62 [95 % CI 0·49–0·78]) and overall survival (HR 0·68 [95 % CI 0·52–0·88]) were statistically superior with the addition of Atezolizumab. Albeit from an interim analysis, the overall survival improvement was observed in spite of the better-than-expected performance of the standard arm as compared to historical data, with a median overall survival of 23 months versus 17·5 months in the GOG 240 study. Among key secondary endpoints such as overall response rates, the trial results were consistent and favored the experimental arm. The safety profile of the experimental arm was as expected, with a low incidence of treatment suspension due to immune-related adverse events or fistulae.
In the setting of progressive disease after platinum-containing systemic therapy, EMPOWER Cervical 1 (12) is an open-label, multicenter, phase 3 trial which compared the anti PD-1 checkpoint inhibitor cemiplimab against the investigator’s choice of standard chemotherapy, regardless of PD-L1 expression. Among 608 immunotherapy-naïve patients, cemiplimab was superior to chemotherapy in terms of OS, with a HR 0.69 (95 % CI 0.56 to 0.84). Median overall survival with cemiplimab was 12.0 months as compared to 8.5 months, and patients on the experimental arm benefited from superior objective response rates: 16.4 % versus 6.3 %. Furthermore, quality of life during treatment was superior with immunotherapy with regards to the standard arm (13).
In conclusion, immunotherapy constitutes a valuable and effective addition to the current standard of care in the management of advanced cervical cancer, either combined upfront to platinum-based chemotherapy in association with bevacizumab, or as a single agent in second line therapy after a platinum-backbone regimen.
Additional treatment options: Antibody-drug conjugates enter the stage
Antibody-drug conjugates (ADCs) have shown remarkable potential in the treatment of several cancers (14). Tisotumab vedotin (TV) is designed to target tissue factor (TF) and locally deliver its attached chemotherapy payload. Among cervical cancers, TF is expressed abnormally and is linked to a negative prognosis, indicating its potential as a therapeutic target. The toxicity profile of TV is manageable and mainly comprised of ocular toxicity (conjunctivitis, dry eye and reversible keratopathy), peripheral neuropathy and minor bleeding events.
In the relapsed setting, the phase III study InnovaTV-301 (15) compared this novel agent to investigator’s choice of chemotherapy after failure of first line treatment, including previous upfront quadruplet therapy with pembrolizumab in a third of the trial population. With 502 patients enrolled and a median follow-up of 10.8 months, treatment with TV resulted in a 30 % reduction in the risk of death as compared to chemotherapy (HR 0.70; [95 % CI 0.54-0.89)) with significantly longer median OS (11.5 months versus 9.5 months). Confirmed ORR was 17.8 % and 5.2 % in the TV and chemotherapy arms, respectively. Adverse events were consistent with its safety profile, with no new safety signals. Discontinuation due to toxicity was 5.6 % among all treatment arms.
These findings sparked the interest for a potential synergy between TV and other agents. The open-label, multicenter phase Ib/II InnovaTV-205 trial (16) explored TV in combination with either carboplatin, pembrolizumab, or bevacizumab in recurrent or metastatic cervical carcinoma patients deemed ineligible for standard treatments. The three doublets showed an ORR of 35 % and a duration of response of 14 months, results that constitute a noteworthy improvement as compared to historical results with single agent chemotherapy.
Another promising, tumor-agnostic strategy is targeting HER2 overexpression, which is present in approximately 5 % of advanced cervical cancer cases. DESTINY-PanTumor-02 (17) evaluated treatment with the ADC Trastuzumab-deruxtecan among a wide range of solid tumours, including cervical cancer in progression after at least one systemic treatment. It is worth noting that HER2 expression was defined according to the pathological standards for gastric cancer. The primary end point was investigator-assessed objective response rate (ORR). Among 40 cervical cancer patients, ORRs were 50.0 % for the whole cohort (95 % CI, 33.8 to 66.2), with a remarkable 75 % response rate in patients with the highest HER2 expression (IHC 3+). Median and 12-month PFS in this cohort was 7 months and 29 %, respectively. The observed safety profile was consistent with previous trials in other disease settings, including the incidence of interstitial lung disease.
Final Conclusions
After decades of limited advancements in the field of cervical cancer, we are finally entering a new era where more treatment options are available to improve the outcome of patients with cervical cancer, both in the locally advanced and metastatic settings. The identification of predictive biomarkers is needed to further improve patients’ selection and spare from unnecessary toxicities, patients who will not benefit from these agents.
Copyright Aerzteverlag medinfo AG
Oncology Institute of Southern Switzerland (IOSI)
Ente Ospedaliero Cantonale (EOC)
6500 Bellinzona
Oncology Institute of Southern Switzerland (IOSI)
Ente Ospedaliero Cantonale (EOC)
6500 Bellinzona
Oncology Institute of Southern Switzerland (IOSI)
Ente Ospedaliero Cantonale (EOC)
6500 Bellinzona
Die Autor/-innen haben keine Interessenkonflikte im Zusammenhang mit diesem Artikel deklariert.
- In the treatment of locally advanced cervical cancer, the introduction of immunotherapy represents an emerging addition to the currently available standard approach.
- Immunotherapy has shown to be a valuable addition in the recurrent or metastatic setting, either combined upfront with platinum-based chemotherapy and bevacizumab, or as a single agent in second line therapy after receiving a platinum-backbone regimen.
- Antibody-drug conjugates (ADCs) against Tissue factor and HER2 show remarkable potential in the relapsed and metastatic setting, further adding to the evolving treatment landscape.
1. Ronco G, Dillner J, Elfström KM et al. Efficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. Lancet 2014; 383: 524–532.
2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries [published correction appears in CA Cancer J Clin. 2020 Jul;70(4):313]. CA Cancer J Clin. 2018;68(6):394-424. doi:10.3322/caac.21492
3. Pötter R, Tanderup K, Schmid MP, et al. MRI-guided adaptive brachytherapy in locally advanced cervical cancer (EMBRACE-I): a multicentre prospective cohort study. Lancet Oncol. 2021;22(4):538-547. doi:10.1016/S1470-2045(20)30753-1
4. Sturdza A, Pötter R, Fokdal LU, et al. Image guided brachytherapy in locally advanced cervical cancer: Improved pelvic control and survival in RetroEMBRACE, a multicenter cohort study. Radiother Oncol. 2016;120(3):428-433. doi:10.1016/j.radonc.2016.03.011
5. Mileshkin LR, Moore KN, Barnes EH, et al. Adjuvant chemotherapy following chemoradiotherapy as primary treatment in locally advanced cervical cancer vs. chemoradiotherapy alone (OUTBACK): an international, open-label, randomised, phase 3 trial. Lancet Oncol. 2023;24(5):468-482. doi:10.1016/S1470-2045(23)00147-X
6. McCormack M, et al. A randomised phase III trial of induction chemotherapy followed by chemoradiation compared with chemoradiation alone in locally advanced cervical cancer. The GCIG INTERLACE trial. ESMO Congress 2023, LBA8
7. Monk BJ, Toita T, Wu X, et al. Durvalumab versus placebo with chemoradiotherapy for locally advanced cervical cancer (CALLA): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2023;24(12):1334-1348. doi:10.1016/S1470-2045(23)00479-5
8. Lorusso D, Xiang Y, Hasegawa K, et al. Pembrolizumab or placebo with chemoradiotherapy followed by pembrolizumab or placebo for newly diagnosed, high-risk, locally advanced cervical cancer (ENGOT-cx11/GOG-3047/KEYNOTE-A18): a randomised, double-blind, phase 3 clinical trial. Lancet. 2024;403(10434):1341-1350. doi:10.1016/S0140-6736(24)00317-9
9. Tewari KS, Sill MW, Long HJ 3rd, et al. Improved survival with bevacizumab in advanced cervical cancer [published correction appears in N Engl J Med. 2017 Aug 17;377(7):702]. N Engl J Med. 2014;370(8):734-743. doi:10.1056/NEJMoa1309748
10. Monk BJ, Colombo N, Tewari KS, et al. First-Line Pembrolizumab + Chemotherapy Versus Placebo + Chemotherapy for Persistent, Recurrent, or Metastatic Cervical Cancer: Final Overall Survival Results of KEYNOTE-826. J Clin Oncol. 2023;41(36):5505-5511. doi:10.1200/JCO.23.00914
11. Oaknin A, Gladieff L, Martínez-García J, et al. Atezolizumab plus bevacizumab and chemotherapy for metastatic, persistent, or recurrent cervical cancer (BEATcc): a randomised, open-label, phase 3 trial. Lancet. 2024;403(10421):31-43. doi:10.1016/S0140-6736(23)02405-4
12. Tewari KS, Monk BJ, Vergote I, et al. Survival with Cemiplimab in Recurrent Cervical Cancer. N Engl J Med. 2022;386(6):544-555. doi:10.1056/NEJMoa2112187
13. Oaknin A, Monk BJ, Vergote I, et al. EMPOWER CERVICAL-1: Effects of cemiplimab versus chemotherapy on patient-reported quality of life, functioning and symptoms among women with recurrent cervical cancer. Eur J Cancer. 2022;174:299-309. doi:10.1016/j.ejca.2022.03.016
14. Shastry M, Gupta A, Chandarlapaty S, Young M, Powles T, Hamilton E. Rise of Antibody-Drug Conjugates: The Present and Future. Am Soc Clin Oncol Educ Book. 2023;43:e390094. doi:10.1200/EDBK_390094
15. Vergote IB, Gonzalez MA, Fujiwara K, et al: innovaTV 301/ENGOT-cx12/GOG-3057: A global, randomized, open-label, phase III study of tisotumab vedotin vs investigator’s choice of chemotherapy in 2L or 3L recurrent or metastatic cervical cancer. ESMO Congress 2023. Abstract LBA9. Presented October 22, 2023.
16. Vergote I, Van Nieuwenhuysen E, O’Cearbhaill RE, et al. Tisotumab Vedotin in Combination With Carboplatin, Pembrolizumab, or Bevacizumab in Recurrent or Metastatic Cervical Cancer: Results From the innovaTV 205/GOG-3024/ENGOT-cx8 Study. J Clin Oncol. 2023;41(36):5536-5549. doi:10.1200/JCO.23.00720
17. Meric-Bernstam F, Makker V, Oaknin A, et al. Efficacy and Safety of Trastuzumab Deruxtecan in Patients With HER2-Expressing Solid Tumors: Primary Results From the DESTINY-PanTumor02 Phase II Trial. J Clin Oncol. 2024;42(1):47-58. doi:10.1200/JCO.23.02005
info@onco-suisse
- Vol. 14
- Ausgabe 6
- September 2024