- Whoops-Resections – how can we improve?
Eine Whoops-Resektion entspricht einer inadäquat, sprich ohne präoperative radiologische und bioptische Diagnostik, geplanten und konsekutiv durchgeführten Resektion eines Tumors, in deren Rahmen postinterventionell überraschenderweise die Diagnose eines bösartigen Tumors gestellt wird. Solche ungeplanten Tumorresektionen treten regelmässig (bis zu 50%) bei Sarkomen auf, sind mit einer erhöhten Lokalrezidivrate und möglicherweise ungünstigerer Prognose assoziiert. Mit kurativem Therapieziel besteht in der Regel die Indikation zur ausgedehnten Nachresektion, Bestrahlung und ggf. Chemotherapie. Die Folge ist ein grösserer Operationssitus, eine erhöhte Rate an posttherapeutischen, funktionellen Einschränkungen und somit Verminderung der Lebensqualität. Mit diesem Artikel bezwecken wir auf die Problematik von Whoops-Resektionen aufmerksam zu machen und Richtlinien zu dessen Verhinderung zu formulieren.
A whoops resection corresponds to an inadequately planned and consecutively performed resection of a tumor, i.e. without preoperative radiological diagnostics and biopsy, in the course of which the diagnosis of a malignant tumor is surprisingly made postoperatively. Such unplanned tumor resections occur regularly (up to 50%) in sarcomas, are associated with an increased local recurrence rate and possibly an unfavorable prognosis. With curative therapy goals, there is usually an indication for extensive resection, radiation and possibly chemotherapy. The consequence is an extensive surgical site, an increased rate of post-therapeutic, functional limitations and thus a reduction in the quality of life. The purpose of this article is to draw attention to the problem of whoops resections and to formulate guidelines for their prevention.
Key Words: sarcoma, unplanned excision, Whoops, surgery, prognosis
Soft tissue sarcomas (STS) are a rare and heterogeneous group of malignant tumors of mesenchymal origin that comprise less than 1 percent of all adult malignancies and approximately 12 percent of pediatric cancers. The age-standardized incidence rate in Switzerland is 4.43 per 100,000 person-years for STS. Sarcomas occur at all anatomic body sites, but the majority are in the extremities (Fig.1) (1).
Due to their rarity, STSs are often not considered in the differential diagnosis. Often, STS presents as a slowly enlarging, superficial, soft-tissue lesion, sometimes following a history of trauma. Given the much higher frequency of benign soft-tissue lesions, STSs are often treated surgically as unplanned excisions (UE). Hence, excision is performed without previous imaging and biopsy, without adherence to a proper diagnostic pathway and therapeutic plan. As both the surgeon and the patient are astonished to note the pathological finding of a supposedly benign lesion as “malignant”, the surgery performed is referred to as a “Whoops resection”. Consecutively, micro- or even macroscopically residual tumor might persist in the surgical situs, potentially requiring second and maybe more extensive surgery (including amputation). As a result, UE might lead to increased functional disabilities and therefore reduced quality of life with a relevant impact on daily living. Importantly, UE might be associated with increased local recurrence rates and decreased survival (2).
The rates of whoops resections reported in the literature vary from approximately 11.3% to over half of STS resections (3). While there appears to be no association with patient residence and insurance status, UE rates are lower in tertiary centers than in non-tertiary hospitals. Notably, these rates highly depend on the country-specific structure and collaboration within sarcoma networks and referral patterns.
According to current guidelines, biopsy-proven STS requires a wide surgical resection as an essential treatment for virtually all patients. Depending on patient and tumor characteristics, perioperative radiation- and/or chemotherapy should be considered in order to improve patient outcome.
This review aims to raise awareness for this clinically relevant topic, discuss the impact of UE on patient quality of life and treatment outcomes, and provide guidance for future improvements.
Diagnosis of STS
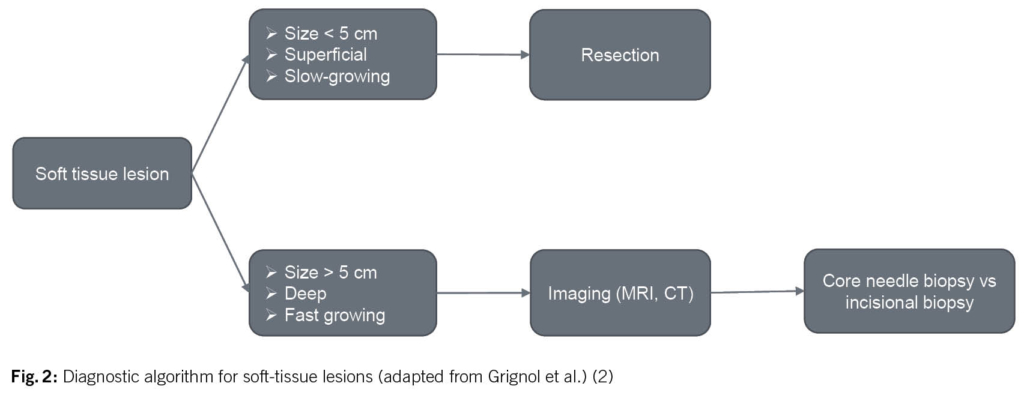
Given that malignant soft tissue sarcomas are at least 100 times less common than benign soft tissue lesions, the decision whether a soft tissue mass warrants further evaluation before direct surgical excision might be difficult. In order to prevent unplanned and inappropriate tumor resection, the initial evaluation of a patient with a suspected STS begins with the clinical history and a proper physical examination. Based on the results of a prospective review of 365 patients with confirmed STS, a tumor size of > 5 cm, deep tumor location and a history of rapid growth seem to be the most relevant parameters warranting further diagnostic investigations (Fig. 2)(4).
If a malignant diagnosis is suspected, i.e. the above criteria are fulfilled, the first step is to arrange for adequate imaging. Radiographic imaging is used to assist in defining the etiology of a soft tissue mass, determining the extent of a primary tumor for surgical planning, and establishing the presence or absence of metastatic disease. The gold-standard imaging modality for evaluation of soft tissue masses in the extremities, trunk and head and neck region is a magnetic resonance imaging (MRI). The general approach to evaluate for metastatic disease is performing a computed tomography (CT). If imaging continues to show a suspicion of sarcoma, histological examination is essential as the next step for further treatment planning. The preferred method of obtaining tissue is with a core needle biopsy (≥ 14-16 G needles). This biopsy should be planned by the surgeon who will be responsible for the resection in collaboration with the radiologist. In case an incisional biopsy is necessary in order to obtain an adequate tissue block for diagnosis based on immunohistochemical or molecular analysis, it is important that the intervention is performed by a surgeon who will be responsible for the definitive resection. This will ensure that the biopsy site is planned according to natural anatomical borders, so the scar may be resected en bloc at the time of definitive surgery to prevent tumor cell dissemination (2).
Impact of Whoops resection on functional and disease outcome
a) Local recurrence
In a review by Grimer et al. the rates of residual tumors at additional excision after UE varied from 31% to 72% (5). Consequently, the risk of developing local recurrence after UE seems to be increased. The local control rate of adequately treated STS (wide resection plus/minus radiation therapy) is reported to be approximately 85-90% and 90-100% for high-grade and low-grade STS, respectively. The local recurrence rate after UE and wide re-excision in the available literature at 5 years ranges from 5% to 45%, with an average of 14%. Of note, there is a clear relationship between grade, residual tumor, and margin status on the risk of local recurrence in the reported series.
b) Survival
Whether UE have an impact on survival is still a matter of debate. In general, no detrimental effect on survival could be shown so far in this particular clinical scenario. However, patients who undergo UE tend to present with superficial tumor site, smaller tumor size and therefore, earlier stage disease, which again corresponds to a better overall prognosis and might bias the interpretation of outcome analysis.
c) Function and quality of life
Furthermore, there is no doubt that patients undergoing re-excision have a greater number of operations and are more likely to need plastic and reconstructive surgery than those having primary resections. Radiation therapy and plastic and reconstructive surgery involvement in this setting are reported to be independently associated with wound complications. It is a matter of fact that UE and the necessary additional salvage treatments are associated with a worse functional outcome and hence reduced quality of life.
Management of Whoops resection
Surgery
A complete surgical resection remains the mainstay of any STS treatment. The tumor mass must be removed en bloc through normal uninvolved tissue outside of the pseudocapsule, considered as “safety distance”. In the case of a whoops lesion, the treating surgeon does not think of the possibility of a sarcoma. Therefore, assuming a benign lesion, the principles of correct STS surgery are not followed, and the mass is resected intralesional within its pseudocapsule. This explains the high risk of residual tumor cells in the surgical area. A salvage wide resection of the unplanned STS excision site is usually the requirement for curative treatment. En bloc removal of the entire prior operative site, including the surgical scar and drain tracts, as well as appropriate margins of adjacent uninvolved soft tissue to fully wrap the prior STS location is necessary. The estimation of the surgical area contaminated by tumor cells based on clinical examination and imaging imposes a big challenge. This uncertainty is countered with even larger safety margins than usual. Furthermore, contamination of adjacent, previously uninvolved tissues from the use of transverse incisions, drains placed outside of the line of resection, postoperative hematoma that violate tissue planes, and leakage of tumor pieces must be considered in the salvage procedure. All the mentioned points lead to an increased surgical and functional morbidity compared to what would have been appropriate for the STS at initial presentation before the unplanned excision.
Despite the risk of long-term functional impairment, extensive surgery is justified as it provides better local control than non-surgical treatment alone (5-year local recurrence free survival: 87.9% vs. 49.9%)(6).
Plastic and Reconstructive Surgery
The extent of soft tissue reconstruction after wide re-resection is reported in the literature in 47-89% of the patients, while the highest rates of flap reconstruction needed in planned excisions of STSs vary between 33-47% in specialized sarcoma centers (5). The ensuing defect is usually bigger than in a case of planned wide resection due to ill-defined margins of the tumor that could theoretically guide the surgeon during primary resection, the presence of distant drains placed outside the operated region with subsequent contamination of the drain tracts, postoperative extensive hematomas that taint surrounding healthy tissue, as well as the position of the initial scar. In these cases, free tissue transfers, together with regional pedicled flaps are the optimal choice for coverage, while local flaps are usually avoided. Donor site morbidity is generally not a problem after perforator flaps, such as the anterolateral thigh flap, but when a latissimus dorsi musculocutaneous flap has to be raised, decreased range of motion with difficulties during daily living and sports activities have been reported in up to 41% of the patients (7).
Radiation-Oncology
There is evidence from randomized trials that in patients with STS treated with planned surgery, the addition of perioperative external beam radiation therapy (RT) significantly improves local control (95% vs 70%). In preoperative RT, only a moderate radiation dose is required. Furthermore, in this setting less normal tissue is exposed to radiation compared to the situation when RT is applied postoperatively. Thus, long-term functional outcome is better if RT is applied preoperatively.
Evidence supporting the use of RT following UE of STS is obviously much thinner. It seems plausible that RT may sterilize sarcoma cells contaminating normal tissue following UE. There is retrospective data revealing a fantastic local control rate (86% at 10 years) in patients undergoing high-dose RT as the only adjuvant treatment after UE (because further surgery was not feasible).Highest local control rates (95% at 5 years) following UE were reported in a retrospective series (n=44) treated with a median radiation dose of 50 Gy prior to definitive resection (10). Additionally, adjuvant RT after re-resection is reported to be associated with a reduced local failure rate. Until we have better evidence, neoadjuvant radiotherapy followed by definitive oncologic surgery can be considered a standard local approach for STS following whoops surgery.
Chemotherapy
There is currently no clear evidence that ‘’neo-adjuvant or adjuvant chemotherapy’’ has any beneficial effect following a whoops resection of sarcomas. However, some studies suggest that chemotherapy might have an effect on metastatic disease-free survival. The 1997 meta-analysis on adjuvant chemotherapy demonstrated an effect of adjuvant chemotherapy in the group of marginal/involved resection (8). On the other hand, Morii et al. found that only additional wide resection improves oncological outcomes. It is clear that a UE is not equivalent to an R1 resection (6). UE carries a higher risk of local recurrence because adjacent structures become contaminated, which may make oncologic R0 resection impossible. Chemotherapy cannot compensate for R1 or R2 resection and therefore re-resection should always be considered (9). Still, data from retrospective single center studies that examined the postoperative short- and long-term follow-up period in patients lacks any identified prognostic markers for overall survival. Furthermore, these retrospective analyses are inevitably associated with both systematic and random biases, particularly the bias of individual-level data on all patients. In addition, little is known about the impact of the time lag between whoops resection and referral of patients to specialized centers.
Therefore, the use of ‘’neo-adjuvant or adjuvant chemotherapy” should be based on the tumor subtype, localization and risk factors and should be considered especially in high-risk STS. To date, nomograms such as Sarculator and Persarc widely used for identifying high risk sarcomas do not consider whoops resection as a risk factor making their use for primary disease riddled with conflicting results and low-grade evidence. Discussion in specialized centers is crucial since clinical characteristics and histopathological features should be retrospectively reviewed and analyzed with treatment modalities for each case to provide a personalized decision.
Conclusions and recommendations
Although the topic of “whoops resections” is regularly discussed among sarcoma experts, who are involved too late in the diagnostic and therapeutic pathway, it has not yet been possible to substantially reduce the rate of inadequate resections. The main problems seem to be dissemination of knowledge and vigilance in the medical community. In addition, surgical guidelines may reflect commonly encountered problems and neglect extremely rare situations.
Highly Specialized Medicine, as a strategy to pool expertise in specific surgical areas, misses one of the challenges in sarcoma diagnosis and therapy: the first diagnostic steps are initiated and performed outside the centers of excellence, precisely because no thought is given to the differential diagnosis of STS.
Improvement may be achieved by continuously raising awareness of STS among general surgeons, general practitioners and private practices, and by better adherence to preoperative imaging and referral guidelines.
To prevent UE, the European Society of Medical Oncology guidelines recommend that all patients with an unexplained deep mass or superficial soft tissue lesion > 5 cm be referred to a specialist.
Dr. med. Rahel Jost 1
PD. Dr. med. Daniel A. Müller 2
PD. Dr. med. Ioana Lese 3
Dr. med. Stefan Brodmann 4
Dr. med. Antonia Digklia 5
PD Dr. med. Christian Rothermundt 6
PD Dr. med. Attila Kollàr 1
1 Department of Medical Oncology, Inselspital, Bern University Hospital, Bern, Switzerland
2 Department of Orthopaedic Surgery, Balgrist University Hospital, Zürich, Switzerland
3 Department of Plastic and Hand Surgery, Inselspital University Hospital Bern, University of Bern, Switzerland
4 Department of Radiation Oncology, Kantonsspital Winterthur, Switzerland
5 Oncology Department, Centre Hospitalier Universitaire Vaudois (CHUV), Lausanne University, Lausanne, Switzerland.
6 Department of Medical Oncology and Hematology, Kantonsspital St. Gallen, St. Gallen, Switzerland.
Copyright bei Aerzteverlag medinfo AG
Department of Medical Oncology, Inselspital, Bern University Hospital, Bern, Switzerland
Department of Medical Oncology, Inselspital, Bern University Hospital, Bern, Switzerland
Der Autor hat keine Interessenkonflikte im
Zusammenhang mit diesem Artikel deklariert.
◆ Whoops resections are not uncommon.
◆ Standard treatment is wide re-resection, usually combined with radiotherapy.
◆ Residual tumor can be detected in app. 50% of reported cases.
◆ The presence of residual disease is an adverse prognostic factor.
◆ In all lumps bigger than a golf ball proper diagnostic pathway including imaging and biopsy should be performed prior to excision.
1. Kollar A, Rothermundt C, Klenke F, Bode B, Baumhoer D, Arndt V, et al. Incidence, mortality, and survival trends of soft tissue and bone sarcoma in Switzerland between 1996 and 2015. Cancer Epidemiol. 2019;63:101596.
2. Grignol VP, Lopez-Aguiar AG. The Implications of an Unplanned Sarcoma Excision (the “Whoops” Operation). Surg Clin North Am. 2022;102(4):529-38.
3. Melis AS, Vos M, Schuurman MS, van Dalen T, van Houdt WJ, van der Hage JA, et al. Incidence of unplanned excisions of soft tissue sarcomas in the Netherlands: A population-based study. Eur J Surg Oncol. 2022;48(5):994-1000.
4. Hussein R, Smith MA. Soft tissue sarcomas: are current referral guidelines sufficient? Ann R Coll Surg Engl. 2005;87(3):171-3.
5. Grimer R, Parry M, James S. Inadvertent excision of malignant soft tissue tumours. EFORT Open Rev. 2019;4(6):321-9.
6. Morii T, Yabe H, Morioka H, Anazawa U, Suzuki Y, Toyama Y. Clinical significance of additional wide resection for unplanned resection of high grade soft tissue sarcoma. Open Orthop J. 2008;2:126-9.
7. Lee KT, Mun GH. A systematic review of functional donor-site morbidity after latissimus dorsi muscle transfer. Plast Reconstr Surg. 2014;134(2):303-14.
8. Adjuvant chemotherapy for localised resectable soft-tissue sarcoma of adults: meta-analysis of individual data. Sarcoma Meta-analysis Collaboration. Lancet. 1997;350(9092):1647-54.
9. Alsina AC, Sacchetti F, Kaya H, Yaman B, Tamsel I, Sabah D. Impact of the unplanned excision on the oncological outcomes of patients with soft tissue sarcomas: a single-center retrospective review of 490 patients. Acta Orthop Traumatol Turc. 2022;56(4):272-7.
10. Jones DA, Shideman C, Yuan J, Dusenbery K, Manivel CJ, Ogilvie C, et al. Management of unplanned Excision for soft-tissue sarcoma with preoperative radiotherapy followed by definitive resection. Am J Clin Oncol. 2016:39:586-592.
info@onco-suisse
- Vol. 13
- Ausgabe 3
- Mai 2023