- Too Much of a Good Thing: Severe Hypercalcemia Presenting with Lethargy and Kidney Failure
Zu viel des Guten: Schwere Hyperkalzämie mit Lethargie und Nierenversagen
Zusammenfassung: Wir präsentierten einen Fall eines 58-jährigen Patienten mit einer Vorgeschichte einer Laryngo-Pharyngektomie inklusive bilateraler Thryroidektomie aufgrund eines Hypopharynxkarzinoms, welcher sich mit Lethargie, akutem Nierenversagen und schwerer Hyperkalzämie präsentiert. Das Milk Alkali Syndrom wurde aufgrund des deutlich erhöhten Kalziums und der anamnestischen Vitamin-D Supplementierung nach Ausschluss anderer Ursachen diagnostiziert. Nach der initialen Therapie mit NaCl, Furosemid und Denosumab entwickelte der Patient eine symptomatische schwere Hypokalzämie als unerwünschte Arzneimittelwirkung von Denosumab.
Schlüsselwörter: Hyperkalzämie, Milch-Alkali Syndrom, Denosumab, Nierenbiopsie
Case Presentation
A 55-year-old man was referred to the intensive care unit because of lethargy, acute kidney failure and hypercalcemia.
On the day of admission, the patient was not able to rise from a chair and fell to the floor. Emergency medical service suspected a stroke and admitted the patient to the stroke unit. After ruling out a cerebrovascular event, the patient was diagnosed with severe symptomatic hypercalcemia and kidney failure and admitted to the intensive care unit for treatment and diagnostic workup.
The current medication on admission is listed in Table 1.
The patient has a history of laryngo-pharyngectomy including bilateral thyroidectomy due to hypopharyngeal squamous cell cancer. Due to low calcium levels on routine follow-up appointments, the calcium supplementation dose was increased several weeks before the current presentation. The patient’s wife reported a three-week history of progressive fatigue, confusion, somnolence, muscle weakness and gait instability. Unintended weight loss, fever or night sweats, dysuria, polyuria, oliguria, arthralgia, or dermatologic symptoms were not reported.
On examination, the patient was afebrile with a temperature of 36.4°C, a blood pressure of 138/91 mmHg, a heart rate of 102/min, oxygen saturation of 88% at room air and a body mass index of 23.7 kg/m2. The patient was lethargic with a Glasgow Coma Scale (GCS) of 11 points (E4, V2, M5). Physical examination revealed rhythmic heart sounds without murmurs or rubs, normal bilateral breath sounds on pulmonary auscultation, and dry mucous membranes and skin. Regular bowel movements without tenderness or guarding were observed.
Investigations and Differential Diagnosis
The differential diagnosis of hypercalcemia is broad with cancer and hyperparathyroidism representing the most common causes. While intrinsic kidney failure normally results in hyperphosphatemia with accompanied hypo- or normocalcemia, the kidney failure was interpreted as secondary to hypercalcemia.
A diagnostic algorithm for the evaluation of hypercalcemia is presented in Figure 1.
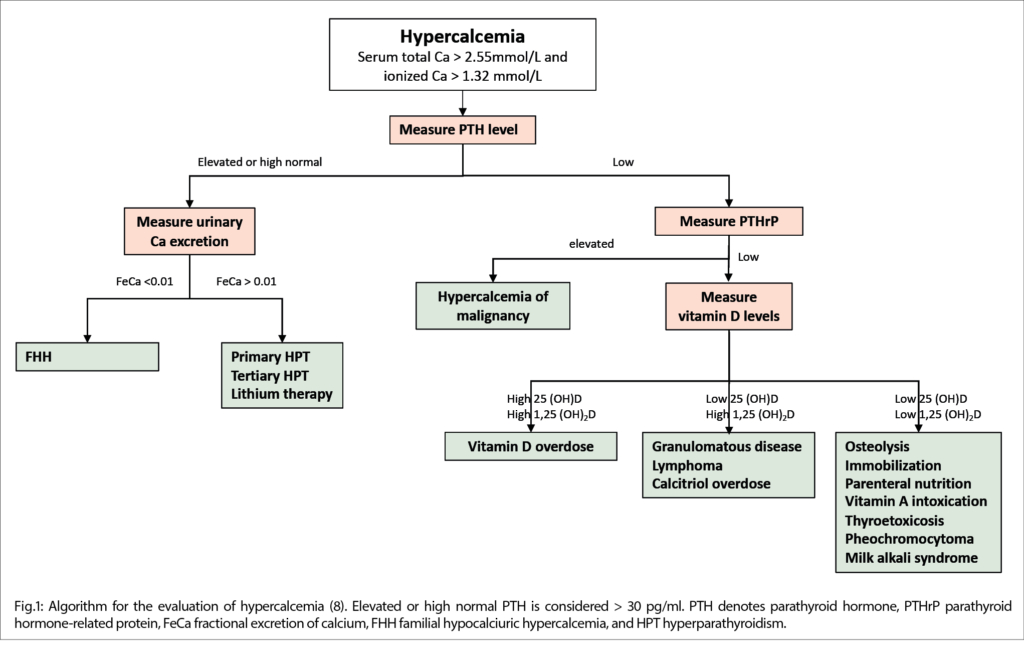
Parathyroid hormone (PTH) was decreased (5.8pg/ml) ruling out hyperparathyroidism. Given the patient’s history of squamous cell cancer, parathyroid hormone-related peptide (PTHrP) was measured, the result of which was below the detection limit (<0.5pmol/L; see Table 2). Elevated 25(OH)-vitamin D (84.1ng/mL) levels and normal levels of 1,25(OH)2-vitamin D (36.3pg/mL) indicate mild vitamin-D overdose and render an autoimmune (e.g., sarcoidosis), infectious (bacterial, mycobacterial, fungal), granulomatous disease or lymphoma unlikely as a diagnosis.
Ultrasonography revealed no evidence of a postrenal cause for acute renal failure. Serological tests for anti-neutrophil cytoplasmic antibodies (ANCA), anti-nuclear antibodies (ANA), rheumatoid factor, HIV, and HBV/HCV were negative. In the absence of typical clinical signs (edema, elevated blood pressure) and given repeated normal urine sediment findings, nephritic or nephrotic kidney disease was ruled out. Serum protein electrophoresis showed no bands and a normal kappa/lambda ratio made multiple myeloma (or a monoclonal gammopathy of renal significance including light and/or heavy chain amyloidosis) unlikely.
Diagnosis and Treatment
As other causes of hypercalcemia were ruled out and given the rare combination of metabolic alkalosis and acute kidney failure, hypercalcemia and elevated 25-OH-vitamin-D levels, a diagnosis of milk-alkali syndrome and concomitant vitamin D intoxication was made as a diagnosis of exclusion. A careful review of the medication on admission showed overdosage of the combined calcium/vitamin D supplement as the underlying cause.
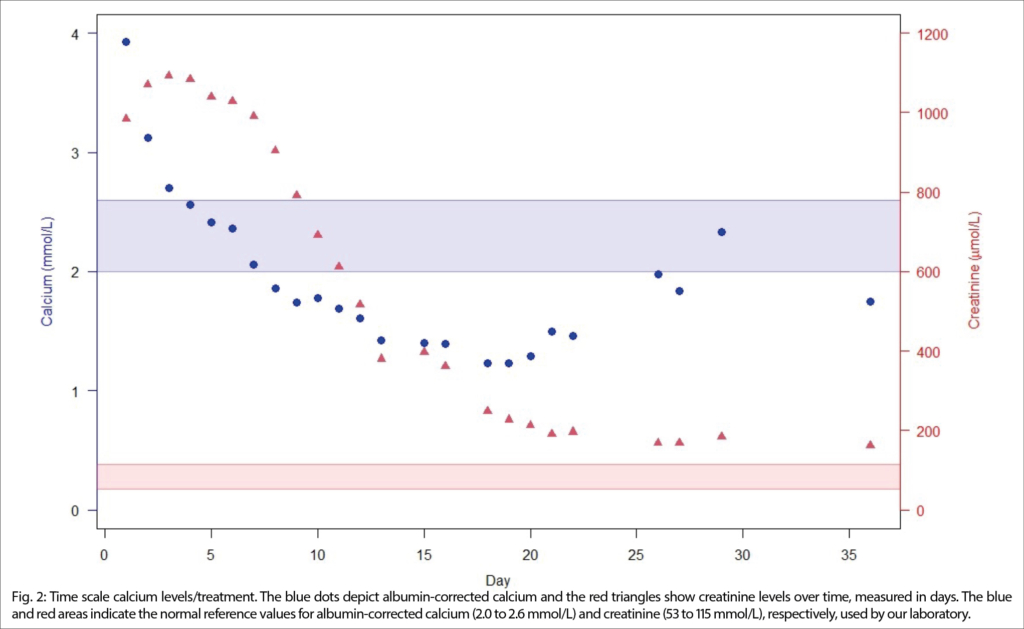
Initially, intravenous isotonic saline and furosemide were started and 120mg denosumab was administered given the patient’s history of cancer and suspected malignant hypercalcemia. Calcium levels normalized over five days. However, on day 8, severe hypocalcemia developed, which didn’t respond sufficiently to renewed calcium supplementation, first orally and then intravenously. Thus, we initiated treatment with oral calcitriol and continued high-dose calcium supplementation. Due to concomitant hypomagnesemia, we supplemented magnesium orally. Parathyroid hormone levels were measured and again very low, confirming primary hypoparathyroidism. Finally, on day 26, calcium levels returned to baseline levels (2.0mmol/L), see Figure 2.
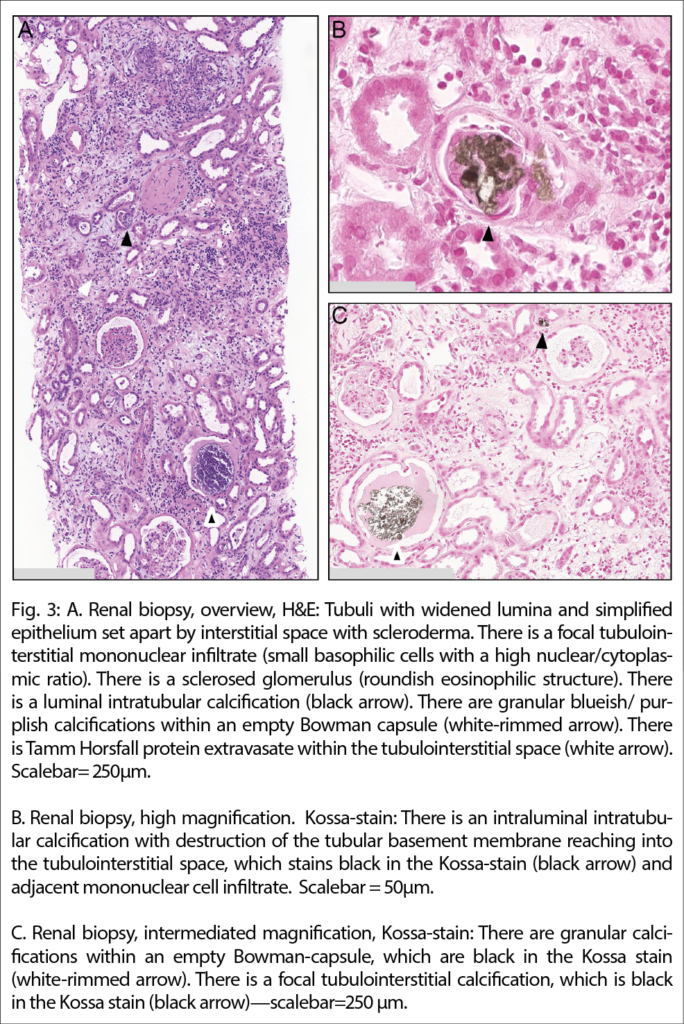
Despite the timely initiation of treatment, kidney failure persisted. Notwithstanding negative urine sediment analysis, a transcutaneous kidney biopsy was performed to definitively rule out nephritic kidney disease and further explore the etiology of the kidney failure. Histologically chronic changes included a few sclerosed glomeruli. Proximal tubular epithelia showed some loss of brush borders and there were regenerative changes consistent with acute tubular injury and regeneration. There were few non-polarizing calcifications consistent with calcium phosphate (basophilic on hematoxylin and eosin stain, and black on the Kossa-stain) and multifocal tubulointerstitial Tamm-Horsfall-protein (uromodulin) extravasates associated with an inflammatory reaction but no typical findings of nephrocalcinosis. Figure 3, Immunofluorescence staining for IgG, IgA, C1q, and kappa and lambda light chains were negative.
After normalization of serum calcium values following hypocalcemia and stable recovery of kidney function, the patient was discharged from hospital care. Given the severe hypocalcemia due to denosumab, we prescribed oral calcium supplements and continued oral calcitriol treatment.
Regular follow-up appointments were arranged for serum electrolyte measurements and kidney function testing in short intervals. After three weeks, the patient redeveloped mild hypercalcemia, indicating the resolution of the denosumab side effect. After adaptation of Calcium supplementation, kidney function remained stable (Figure 2).
Discussion
In 1915, Bertram Sippy developed the “Sippy regimen”, a diet that consisted primarily of milk and cream with a combination of antacids (sodium bicarbonate and magnesium carbonate) for treating peptic ulcer disease. In the subsequent decades, toxic reactions with a syndromic triad including hypercalcemia, metabolic alkalosis and renal failure termed milk-alkali syndrome (MAS) were observed in these patients managed with the Sippy diet (1,2). Historically, three different subtypes of MAS have been described; acute, subacute (Cope‘s syndrome), and chronic (Burnett‘s syndrome).
The syndrome resurged in the late 20th century due to the increased awareness of the morbidity and mortality of osteoporosis and the popularity of over-the-counter vitamin D and calcium supplements (3).
After the establishment of proton pump inhibitors and antihistamines as efficacious therapies for gastrointestinal ulcer disease, Patel et al. suggested the term “calcium alkali syndrome” given the change in the pathophysiology and demographics of the disease from young men with peptic ulcer disease to post-menopausal woman, solid organ transplant recipients, pregnant woman and patients on dialysis (4).
The exact incidence of MAS remains unknown, but previous studies suggested that MAS is an underdiagnosed disease being the third most common cause of hypercalcemia after malignancy and hyperparathyroidism (5).
Several pathophysiological aspects of the development and persistence of MAS in this patient warrant further discussion.
First, hypercalcemia in MAS only develops if the calcium input exceeds the renal calcium excretion and the primary calcium regulatory mechanisms in the kidney, bone and intestine through PTH and calcitriol are exhausted. The required amount of calcium carbonate to induce MAS is reported to be > 4 g/d. The prescribed daily dose of calcium carbonate in our patient was 6.25g. Calcium induces gastric acid production, which further increases the availability of free calcium for absorption in the small intestine.
Second, several conditions need to be fulfilled to maintain hypercalcemia in MAS. Elderly people have decreased bone capacity and impaired renal function to regulate calcium levels. Thiazide diuretics are commonly prescribed antihypertensive drugs, which decrease calcium excretion by inducing volume depletion and enhance passive calcium absorption in the distal convolute tubule. Other drugs associated with an increased risk of MAS are non-steroidal anti-inflammatory drugs (NSAID) and renin-angiotensin-aldosterone system (RAAS) inhibitors.
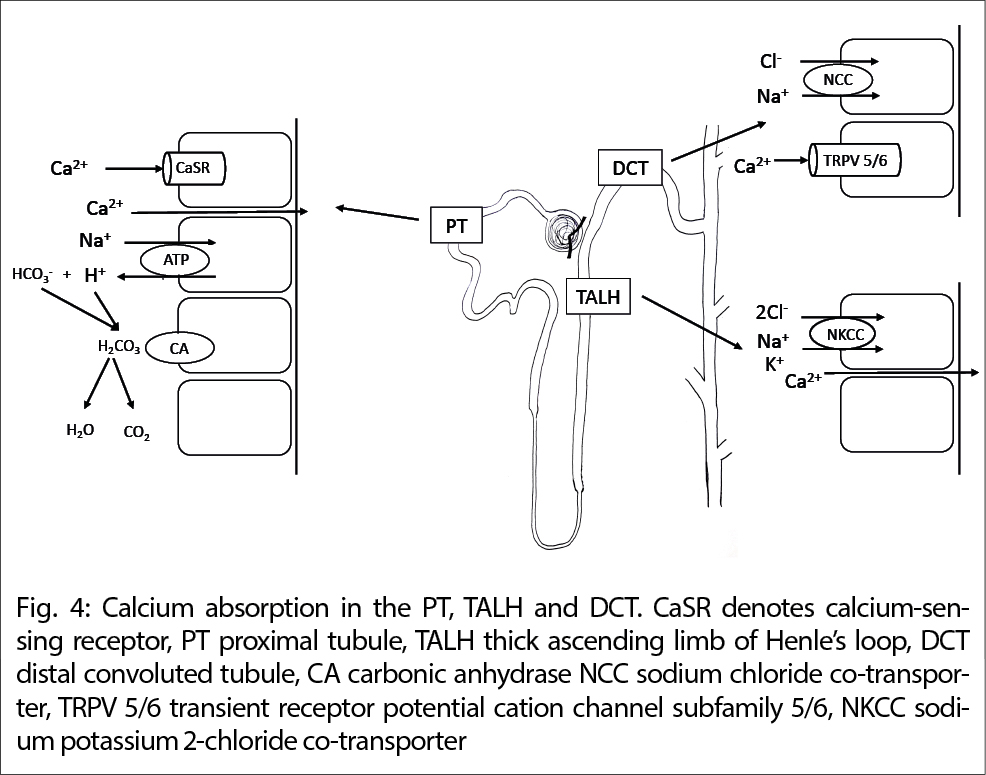
Third, hypercalcemia in MAS suppresses PTH, which increases the activity of carbonic anhydrase in the proximal tubule aggravating metabolic alkalosis. This enhances calcium absorption in the proximal tubule, the loop of Henle and the distal tubule by stimulating the Calcium-sensing receptor (CaSR), the calcium channel TRP V5/6 and increasing transcellular calcium influx. Hypercalcemia causes vasoconstriction of the renal afferent arteriole and impairs the concentrating ability of the renal tubules and the antidiuretic hormone (ADH)-dependent water reabsorption aggravating volume depletion, alkalosis, and kidney injury, see Figure 4.
Fourth, calcium carbonate supplements normally result in hypo-or normophosphatemia, which further increases calcitriol levels, promotes the release of calcium and alkali from the bone and increases intestinal calcium absorption. In our patient with chronic hypoparathyroidism due to radical resection of hypopharyngeal carcinoma, hyperphosphatemia was observed at presentation underyling the concurrent vitamin D overdose and kidney failure, which further increased intestinal and renal calcium absorption. Of note, in most case reports of MAS, PTH and calcitriol are appropriately suppressed.
Given the prolonged kidney failure after calcium correction, a kidney biopsy was performed on this patient. Histological findings showed acute tubular injury and regenerative epithelial cells associated with extravasation of Tamm Horsfall-protein (THP) into the interstitial space and resulting inflammatory reaction as well as few tubulointerstitial calcifications and periglomerular fibrosis.
As the diagnosis of MAS is made clinically, a kidney biopsy is not required to establish the diagnosis. Therefore, published literature on histological findings in this setting is limited. Pathophysiologically, findings consistent with nephrocalcinosis are expected, which is histologically defined as the deposition of calcium phosphate crystals within renal tubules and sometimes within the interstitium (6).
Our case adds to the literature on histological findings on kidney biopsies performed in the clinical setting of MAS. In our case, histologically a degree of chronic tubulointerstitial and glomerular changes was appreciable. In addition, there was acute tubular injury and focal deposition of calcium phosphate crystals, associated with marked tubulointerstitial extravasation of THP and an interstitial inflammatory reaction, a phenomenon, which has been described in the setting of outflow obstruction for which there was no evidence in our patient. As shown in Figure 3a, the intratubular crystalline deposition can injure the tubular wall, thereby arguably eliciting extravasation of THP and a reactive inflammatory response, possibly resulting in interstitial fibrosis and atrophy.
Another important aspect of this case is postoperative hypoparathyroidism, defined as an inappropriately low PTH level in the context of hypocalcemia after neck surgery, which was correctly treated with calcium and vitamin D supplementation. This condition causes a fragile equilibrium in calcium homeostasis and can decompensate easier in acute illness (fever, reduced fluid intake) than in physiological conditions.
Treatment of MAS includes the discontinuation of calcium carbonate, appropriate rehydration and induction of calciuresis with loop diuretics. As malignant hypercalcemia was suspected at initial presentation, denosumab was administered. Treatment-related hypocalcemia after the administration of bisphosphonates or denosumab in MAS is reported and is therefore generally not recommended (7).
In conclusion, we report a case of MAS in a patient with secondary hypoparathyroidism due to radical resection of hypopharyngeal carcinoma presenting with hypercalcemia, acute kidney failure and lethargy. This case highlights that MAS should be considered in any patient presenting with hypercalcemia and metabolic alkalosis, elucidates the pathophysiology and histological findings of MAS and reminds clinicians to thoroughly review the current medication including over-the-counter medicines.
Abbreviations
ADH antidiuretic hormone
ANA anti-nuclear antibodies
ANCA anti-neutrophil cytoplasmic antibodies
CaSR Calcium-sensing receptor
GFR glomerular filtration rate
GCS Glasgow coma scale
MAS milk-alkali syndrome
NCC sodium-chloride cotransporter
NSAID non-steroidal anti-inflammatory drugs
PTH parathyroid hormone
PTHrP parathyroid hormone-related peptide
THP Tamm-Horsfall protein
History
Manuscript submitted: 14.10.2023
Accepted after revision: 25.03.2024
Acknowledgments
The authors would like to thank Dr. Claudia Buetikofer, Dr. Alf Corsenca, and Dr. Stefan Györke for their expertise and contribution to patient care.
Department für Innere Medizin
Kantonsspital Graubünden
Loestrasse 170
7000 Chur
Patrick.hofmann@ksgr.ch
Department of Internal Medicine
University Hospital Zurich
Zurich
Switzerland
arcangelo.carta@usz.ch
Leitender Arzt Innere Medizin und Intensivmedizin
Leiter Intensivstation
Spital Uster AG
Brunnenstrasse 42
Postfach | 8610 Uster
Oberärztin
Institut für Pathologie und Molekularpathologie
Universitätsspital Zürich
Schmelzbergstrasse 12
8091 Zürich
birgitmaria.helmchen@usz.ch
The authors declare no potential conflict of interest related to this article.
- Milk alkali syndrome should be suspected in any patient with hypercalcemia, metabolic alkalosis, and kidney failure.
- A detailed history including antacids, vitamin and calcium-containing supplements is essential to diagnose MAS.
- Treatment includes cessation of calcium intake and rehydration.
- Severe hypocalcemia is a rare adverse effect of denosumab.
1. Burnett CH, Commons RR, Albright F, Howard JE. Hypercalcemia without Hypercalcuria or Hypophosphatemia, Calcinosis and Renal Insufficiency — A Syndrome Following Prolonged Intake of Milk and Alkali. New Engl J Medicine 1949;240:787–94.
2. Zayed RF, Millhouse PW, Kamyab F, Ortiz JF, Atoot A. Calcium-Alkali Syndrome: Historical Review, Pathophysiology and Post-Modern Update. Cureus 2021;13:e13291.
3. Beall DP, Henslee HB, Webb HR, Scofield RH. Milk-Alkali Syndrome: A Historical Review and Description of the Modern Version of the Syndrome. Am J Medical Sci 2006;331:233–42.
4. Patel AM, Adeseun GA, Goldfarb S. Calcium-Alkali Syndrome in the Modern Era. Nutrients 2013;5:4880–93.
5. Patel AM, Goldfarb S. Got Calcium? Welcome to the Calcium-Alkali Syndrome. J Am Soc Nephrol 2010;21:1440–3.
6. Fogo AB, Lusco MA, Najafian B, Alpers CE. AJKD Atlas of Renal Pathology: Nephrocalcinosis and Acute Phosphate Nephropathy. Am J Kidney Dis 2017;69:e17–8.
7. Thongprayoon C, Acharya P, Acharya C, Chenbhanich J, Bathini T, Boonpheng B, et al. Hypocalcemia and bone mineral density changes following denosumab treatment in end-stage renal disease patients: a meta-analysis of observational studies. Osteoporosis Int 2018;29:1737–45.
8. Reagan P, Pani A, Rosner MH. Approach to Diagnosis and Treatment of Hypercalcemia in a Patient With Malignancy. Am J Kidney Dis 2014;63:141–7.
PRAXIS
- Vol. 113
- Ausgabe 4
- April 2024